Experiment 3 - Reduction of a Ketone
Objective
To learn a versatile reaction for the reduction of a ketone (or aldehyde) to an alcohol.Introduction
The carbonyl group (C O) found in aldehydes, ketones, carboxylic acids, esters, amides and other functional groups, plays a major role in determining the chemistry of these functional groups. This is due to the polar nature of the carbon-oxygen bond and to the presence of the relatively weak pi bond.
O) found in aldehydes, ketones, carboxylic acids, esters, amides and other functional groups, plays a major role in determining the chemistry of these functional groups. This is due to the polar nature of the carbon-oxygen bond and to the presence of the relatively weak pi bond.
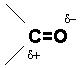
Figure 1
Equation
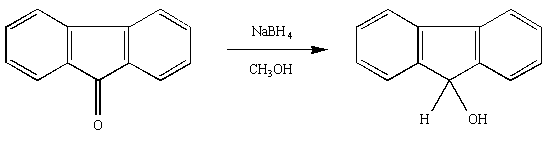
Figure 2
Stoichiometry Note
-
•Check your lecture textbook or online materials for a discussion of the reaction of sodium borohydride. Each of the four hydrides in NaBH4 is "active" and capable of reducing the carbonyl group of a ketone.
-
•How many 'moles' of NaBH4 will be required to reduce one mole of ketone?
Pre-Lab
Complete the pre-lab assignment in WebAssign.Procedure
1
In a 50 mL Erlenmeyer flask, dissolve about 0.500 g (note the actual amount used) of fluorenone in 8-10 mL of 100% methanol.
2
Completely dissolve the solid fluorenone into solution. (Use the warmth from the palm of your hand to help the dissolution process.)
3
Quickly weigh between 0.040 g and 0.060 g of sodium borohydride. (NaBH4 absorbs moisture from the atmosphere; therefore, weigh as rapidly and accurately as possible.)
4
Add the sodium borohydride to the ketone solution in one portion.
5
Swirl vigorously to dissolve.
6
With intermittent swirling, let the reaction mixture stand at room temperature for a period of 15 minutes during which it will turn from yellow to colorless.
7
Add 5 mL of water (solid will form) and heat the reaction mixture to boiling (hotplate setting of 255°C).
8
Occasionally remove the mixture from the hot plate and swirl vigorously.
9
After 5 minutes, remove the mixture from the hot plate and let cool to room temperature. Place the mixture in an ice bath for
10 minutes.
10
Collect the crude 9-fluorenol via vacuum filtration (here is a video that describes how to do the vacuum filtration).
-
•Clamp filter flask to ringstand.
-
•Connect filtration assembly to vacuum.
-
•Put neoprene seal on the mouth of the flask.
-
•Put Buchner funnel in the seal.
-
•Put the filter paper in the funnel.
-
•With vacuum running, squirt 100% methanol on the entire surface of the filter paper to seat paper.
-
•Slowly, pour the solution into the center of the funnel.
-
•If necessary, transfer remaining solid from beaker with a spatula.
-
•For best yield, refilter the filtrate (i.e., the liquid in the filter flask) if it is cloudy.
11
Wash the solid with ice-cold 50% aqueous methanol while still on the vacuum filtration set-up.
12
Transfer solid to a watch glass and allow to dry for 15 minutes.
13
Determine the mass and melting point of the product. Here is a video that shows how to use the DigiMelt apparatus.
14
You will calculate the yield of your synthesis. This video might be useful.
15
Also, determine purity of product as well as presence of unreacted starting material by TLC analysis in 7:3 hexane:acetone. You will have three spots on your TLC plate. One spot will be the starting material, one will be the product you obtained, and one will be fluorenol (which is the expected product). You will prepare a solution of your product by mixing a small amount of the product (just the tip of your microspatula) with 5 mL of 100% methanol.
16
Here is a video that shows how to set up the TLC.
In-Lab Questions
Download and print the worksheet. You will use this worksheet to record your answers to the In-Lab questions.Questions
Record the following data.- Question 1: Amount of fluorenone ___________________ g, ___________________ mol
- Question 2: Amount of sodium borohydride ___________________ g, ___________________ mol
- Question 3: Amount of hydride ___________________ mol
- Question 4: Theoretical yield of 9-fluorenol ___________________
- Question 5: Show calculations for questions 1 - 4.
- Question 6: Actual yield of 9-fluorenol ___________________ g, ___________________ mol
- Question 7: Percentage yield ___________________
- Question 8: Melting point of 9-fluorenol ________________ (observed), ________________ (reported)
- Question 9: Rf values: Fluorenone ___________________ , 9-fluorenol ___________________
- Question 10: Rf values for product ___________________