Experiment 5 - Nitration of Methyl Benzoate
Objective
-
•to demonstrate "Electrophilic Aromatic Substitution"
-
•to provide experience with small-scale synthetic methods
Introduction
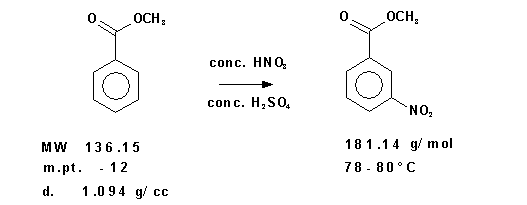
Figure 1
( 1 )
2 H2SO4 + HNO3 → NO2+ + H3O+ + 2 HSO4–Caution:
Concentrated Nitric (HNO3) and Sulfuric Acids (H2SO4) are highly corrosive! Any spills should be promptly flushed with cold water. Discoloration of the skin will result from even a drop of nitric acid.
Concentrated Nitric (HNO3) and Sulfuric Acids (H2SO4) are highly corrosive! Any spills should be promptly flushed with cold water. Discoloration of the skin will result from even a drop of nitric acid.
Pre-Lab
Complete the pre-lab assignment in WebAssign.Procedure
1
Place 1.0 mL of concentrated sulfuric acid into a clean, dry, 6" test tube.
2
Cool sulfuric acid for 10 minutes by swirling in an ice bath (make sure the test tube stays submerged in the ice bath).
3
Add 0.7 mL of methyl benzoate carefully.
4
Shake the mixture to produce one layer. (Do not shake vigorously, just side to side enough to form one layer.)
5
Cool the solution in an ice bath for 5 minutes.
6
In a separate 6" test tube, prepare a mixture of 0.4 mL of concentrated nitric acid and 0.4 mL of concentrated sulfuric acid.
7
Cool this mixture in an ice bath for 10 minutes.
8
Using a Pasteur pipet, add the cold mixture of acids drop by drop to the cold solution of the ester in sulfuric acid. Continuously swirl the ester solution in the ice bath as you add the mixture of acids. Keep the temperature of the reaction at around 0°C throughout the reaction. This should take 5-10 minutes.
Note: Make sure that you keep the reaction cold. If the reaction isn't cold, you will get another product that you cannot filter and purify.
9
After the mixed acids have been added, swirl the test tube in the ice bath for another 5-10 minutes.
10
Finally, allow the reaction mixture to stand at room temperature, with occasional swirling, for another 10 minutes.
11
Fill another 6'' test tube about 1" deep with crushed ice (do not allow water in your test tube). Slowly add the reaction mixture dropwise (via Pasteur pipet) onto the ice with swirling.
12
Allow the ice to melt and then collect the solid material by vacuum filtration on a Buchner funnel. Here is a video that shows how to do vacuum filtration.
13
Carefully rinse the solid on the filter with 0.5-1.0 mL of cool water to remove traces of acid.
14
Carefully rinse the solid with about 0.5 mL of ice-cold 95% ethanol.
15
Allow the crystals to dry by placing them on a watch glass in the oven for 15 minutes.
16
When dry, determine the weight and melting point range of the solid. Here is a video that shows how to determine the melting point.
Waste Disposal
Place the filtrate (the liquid left over from the filtering process) in the 1 L beaker labeled Filtrate Waste.In-Lab Questions
Download and print the worksheet. You will use this worksheet to record your answers to the In-Lab questions.Questions
Record the following data.- Question 1: Amount of methyl benzoate _____________ mL, _____________ g, ______________ mol
- Question 2: Amount of concentrated nitric acid (16 M) ______________ mL, ______________ mol
- Question 3: Amount of concentrated sulfuric acid (18 M) ______________ mL, ______________ mol
- Question 4: Show your calculations.
- Question 5: Theoretical Yield of methyl 3-nitrobenzoate ________________ mol, ________________ g
- Question 6: Actual Yield of product ___________________ g
- Question 7: Percentage Yield ___________________
- Question 8: Melting Point of product (observed) ___________________
- Question 9: Reported Melting Point of methyl 3-nitrobenzoate ___________________