Experiment 6 - Aldol Condensation
Objective
To provide experience with Aldol condensation, a useful reaction to prepare conjugated carbonyl systems.Introduction
Hydrogen atoms that are located on a carbon adjacent (alpha) to a carbonyl group are acidic and can be removed by base. The acidity is due to the fact that the carbanion produced is stabilized by resonance with the carbonyl group (1 and 2). The carbanion is a nucleophile and is capable of adding to electrophilic centers such as the carbon of the carbonyl group in aldehydes and ketones. The result of such a reaction is the formation of a beta-hydroxy carbonyl compound (3). In cases where there are aromatic substituents, these initial products undergo dehydration to yield the conjugated system (4).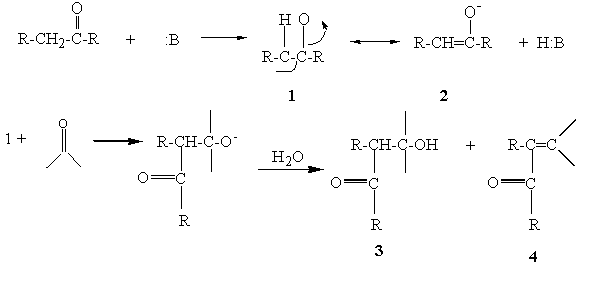
Figure 1
Pre-Lab
Complete the pre-lab assignment in WebAssign.Preparation of Dibenzalacetone

Figure 2
Procedure
1
Mix 2 mL of the sodium hydroxide solution with 2 mL of ethanol in a small Erlenmeyer flask.
Caution:
The NaOH solution is about 20%; avoid contact. Wash hands after use!
The NaOH solution is about 20%; avoid contact. Wash hands after use!
2
Add 0.3 mL of acetone followed by 0.8 mL of benzaldehyde (use dispensing pipets) to the small Erlenmeyer flask.
3
Swirl the flask intermittently for 15 minutes.
4
Collect the product via vacuum filtration. Here is a video that shows how to do the vacuum filtration.
5
Break the suction and carefully pour 20 mL of water over the product. Reapply the vacuum. Repeat this process 3 times. Reapply the vacuum.
6
Wash the precipitate with about 1 mL of cold water followed by about 1 mL of chilled 95% ethanol over the filtration setup.
7
Determine the mass of your crude product and record it on your worksheet; you may need this value in case your recrystallization is unsuccessful.
8
Save a small amount of your crude product in order to determine its melting point.
9
Recrystallize the rest of your sample with ethanol via the following procedure.
-
•Place 200 mL of water in a 400 mL beaker and warm to 60-70°C to make a warm water bath.
-
•Transfer the crude product to a large test tube.
-
•Clamp that large test tube in the bath.
-
•Using a Pasteur pipet, transfer ~3 mL of ethanol to the large test tube containing the crude product and allow to warm to the temperature of the bath (~5 minutes).
-
•With a stirring rod, stir crude product and hot ethanol until solid dissolves.
-
•If all the solid does not dissolve, add 1 mL more of ethanol.
-
•When all solid has dissolved, remove large test tube from bath and allow it to cool slowly to room temperature.
-
•Place in an ice bath to maximize precipitation of product.
-
•Collect crystals via vacuum filtration.
-
•Place crystals on a watch glass in the oven for ~15 minutes to dry.
10
Determine the melting point (here is a
video) of the crude and recrystallized sample, crude yield and percentage yield of the purified product. Here is
a video that can help with the calculations.
In-Lab Questions
Download and print the following worksheet. You will use this worksheet to record your answers to the In-Lab questions.Questions
Record the following data.- Question 1: Amount of benzaldehyde ______________ mL, ______________ g, _______________ mol
- Question 2: Amount of acetone _______________ mL, _______________ g, ________________ mol
- Question 3: Theoretical Yield of Product __________________ mol, __________________ g
- Question 4: Actual Yield of Crude Product ____________________
- Question 5: Percentage Yield of Crude Product ____________________
- Question 6: Melting Point of Crude Product (observed) __________________
- Question 7: Actual Yield of Recrystallized Product __________________
- Question 8: Percentage Yield of Recrystallized Product __________________
- Question 9: Melting Point of Recrystallized Product (observed) __________________
- Question 10: Reported Melting Point for Dibenzalacetone __________________
- Question 11: Record your calculations.
- Question 12: Record the mechanism of the reaction.