Experiment 7 - Preparation of 1,4-diphenyl-1,3-butadiene
Objective
To provide experience with the "Wittig Reaction", one of the most versatile reactions available for the synthesis of an alkene.Introduction
The carbon-carbon double bond, the "Functional Group" of all alkenes, is a very common functionality. It can be prepared in a variety of ways, many of which involve an "Elimination" reaction. For example, alcohols may be dehydrated under the influence of strong acid, or alkyl halides may be dehydrohalogenated in the presence of strong base. Both of these reactions have the disadvantage of employing harsh reaction conditions. In addition, the former reaction frequently results in skeletal rearrangement. A very general procedure for connecting two molecular fragments to make a new and larger molecule was announced in 1954 by the German Chemist Georg Wittig. For this discovery, Wittig was awarded the Nobel Prize in Chemistry in 1979.
Figure 1

Figure 2

Figure 3
Equation
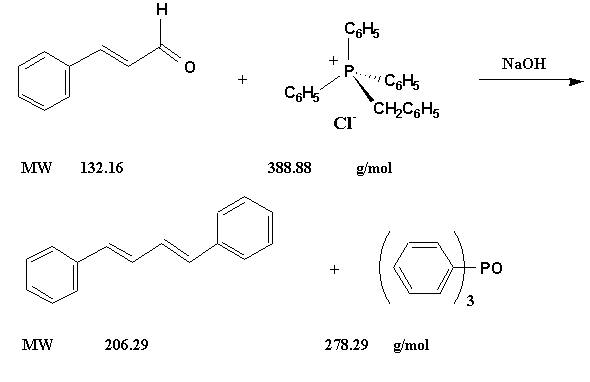
Figure 4
Pre-Lab
Complete the pre-lab assignment in WebAssign.Caution:
Sodium hydroxide is highly corrosive. Rinse any spills with large amounts of cold water. Methylene chloride, or dichloromethane (CH2Cl2), a common component of paint and varnish remover, is a very volatile, toxic compound. Avoid breathing the vapors. Immediately seek fresh air if you should breathe a large amount of the vapor and feel light headed.
Sodium hydroxide is highly corrosive. Rinse any spills with large amounts of cold water. Methylene chloride, or dichloromethane (CH2Cl2), a common component of paint and varnish remover, is a very volatile, toxic compound. Avoid breathing the vapors. Immediately seek fresh air if you should breathe a large amount of the vapor and feel light headed.
Procedure
[adapted from: S. W. Breuer, J. Chem. Educ. 1991, 68, A58-A60.]1
Get a DRY 5 mL conical vial.
2
In order to record the exact weight of benzyltriphenylphosphonium chloride, place the vial on the balance pan, hit the tare button (this should bring the display to zero), and quickly weigh approximately 0.38 g of benzyltriphenylphosphonium chloride into the conical vial. [Keep the supply bottle capped except for the few seconds needed to remove a sample for weighing.]
3
Carefully add three (3) drops of pure cinnamaldehyde to the vial and weigh again.
-
aIf there is not at least 120 mg of the aldehyde, add an additional drop and weigh again.
-
bRecord the mass of the aldehyde.
4
Cap the vial immediately to minimize contact with humid air.
5
Add 1 mL of methylene chloride.
6
Carefully add 0.5 mL of a concentrated NaOH solution to the reaction vial.
Caution:
Concentrated sodium hydroxide (NaOH) is very corrosive. It will burn the skin. Wash any area that contacts the solution with copious amounts of cold water.
Concentrated sodium hydroxide (NaOH) is very corrosive. It will burn the skin. Wash any area that contacts the solution with copious amounts of cold water.
7
Add a magnetic stir vane to the reaction vial, cap the vial, and begin to stir on the magnetic stir plate. DO NOT HEAT! Turn stir rate up until layers are no longer separate while mixing and stir for about 20 minutes after the addition.
8
While the solution is mixing, label three large test tubes "1", "2", and "3".
9
Weigh test tube "3" and record the mass.
10
When stirring is complete, remove the reaction vial from the stir plate, in order to clear the hotplate for the sand bath.
11
Place 25 mL of sand in a 50 mL beaker to create a sand bath.
12
Begin heating the sand bath on the hot plate. Hot plate should be set at 300°C. (Sand warms slowly, so beginning immediately allows sufficient time.)
Caution:
The sand bath will get really hot. You won't be able to tell just by looking at it, so use caution.
The sand bath will get really hot. You won't be able to tell just by looking at it, so use caution.
13
Pour the contents of the reaction vial into test tube "1".
14
Rinse the reaction vial with 1 mL of methylene chloride (CH2Cl2). Add the washing to test tube "1".
15
Rinse the reaction vial with 3 mL of water. Add the washing to test tube "1".
16
Extraction
-
aPipet up the lower layer in test tube "1" and vigorously squirt it through the top layer (i.e., remaining layer) using a long (9") Pasteur pipet.
-
bAllow the layers to separate.
-
cCarefully transfer the organic layer into test tube "2" by using the same Pasteur pipet.
-
dAdd an additional 2 mL of fresh methylene chloride to test tube "1".
-
eRepeat steps a-c for the second extraction.
-
fRepeat steps d and e for the third and final extraction
17
To test tube "2", add enough anhydrous magnesium sulfate to cover the bottom of the test tube.
Note: This removes residual water which is evident when the magnesium sulfate clumps. You have added sufficient magnesium sulfate when there is loose powder present at the bottom of the test tube.
18
Allow test tube "2" to sit for 5-10 minutes without swirling.
19
If solution is cloudy, transfer it through a pipet filter (here is a video that shows you how to make a pipet filter) into test tube "3". Otherwise, just decant it off into test tube "3".
20
Place test tube "3" in the sand bath, add a boiling stick, and carefully evaporate off the methylene chloride while keeping the test tube vertical in the sandbath. Heat until signs of boiling no longer persist and the liquid is gone.
21
Remove the boiling stick and allow test tube "3" to cool to room temperature.
22
Determine the mass of your crude product; you may need this value in case your recrystallization is unsuccessful.
23
Transfer 2 mL of ethanol to test tube "3" and return the test tube to the sand bath.
24
Carefully bring the ethanol to a boil.
25
If all of the solid does not dissolve, add 1 mL of ethanol and return to a boil.
26
Remove it from a hotplate after it has reached boiling.
27
Allow the clear solution to cool slowly for about 5-10 minutes. Light yellow crystals should form in the bottom of the test tube.
28
Place the tube in an ice bath to complete crystallization.
29
Isolate the crystals via vacuum filtration (here is a video that shows you how to do vacuum filtration).
30
Record the weight and melting point of the purified product (here is a video that shows you how to take a melting point). Calculate a percent yield of the recrystallized product.
Waste Disposal
1
The aqueous solution in test tube "1" should be poured into the waste bottle labeled "AQUEOUS WITTIG WASTE".
2
Any solid product should be added to the "SOLID WASTE" container.
3
Melting point capillaries and test tubes should be thrown in broken glass.
In-Lab Questions
Download and print the worksheet. You will use this worksheet to record your answers to the In-Lab questions.Questions
Record the following data.- Question 1: Amount of cinnamaldehyde ____________________ g, ____________________ mol
- Question 2: Amount of Wittig reagent ____________________ g, ____________________ mol
- Question 3: Theoretical Yield of product ____________________ mol, ____________________ g
- Question 4: Actual Yield ______________________
- Question 5: Percentage Yield ______________________
- Question 6: Melting Point ______________________ (observed), ______________________ (reported)
- Question 7: Record your calculations.