- Contents >
- Record-Keeping and Check-In Procedures
Record-Keeping and Check-In Procedures
Forms
Miscellaneous Useful Calculations
Few experiments will involve very complicated calculations. However, most will require several relatively simple computations. You must be able to do these computations rapidly and accurately. Review your General Chemistry material if any of the following calculations appear to be difficult or new to you.
Mass Volume
Very often it will be more convenient to measure reagents by volume. This saves the time of weighing. If the density is known, the volume can be simply converted to mass so that the number of moles may be calculated. The density can be obtained from the container label, a chemical catalog, the MSDS, or from one of the tables in the "Handbook of Chemistry and Physics".
The density ('d') of water is 1 g/cc. The density of acetone is 0.791 g/cc. Thus, one cc of acetone weighs 0.791 g.
Question: What volume must we use if we need 16 g of acetone?
since we had only two signficant figures in the wiehgt ( 16 g)
Question: What mass of acetone is contained in 75.2 mL ? ( 1 cc = 1 mL )
Volume x Density = Mass
75.2 mL x 0.791 g/mL = 59.5 g
NOTE three significant figures in this case
Gram Formula Weight
We will be using molecular formulas throughout this course. You should be able to determine the gram formula weight (or, molecular weight) given the molecular formula of a compound.
Question: What is the gram formula weight of acetone ( C3H6O ) ?
| 3 Carbons | | = 3 x | 12.011 | | = | | 36.033 |
| 6 Hydrogens | | = 6 x | 1.0079 | | = | | 6.0474 |
| 1 Oxygen | | = 1 x | 15.9994 | | = | | 15.9994 |
| | | | | | | 58.0798 |
(Molecular Weights (Gram Formula Weights) are generally reported to only two decimal places.)
Question: What is the gram formula weight of Sodium Dichromate?
When we look up sodium dichromate in a handbook or catalog, we find that it is usually found as the dihydrate ( Na2Cr2O7 ·
2 H2O )
| 2 Sodiums | | = 2 x | 22.9898 | | = | | 459796 |
| 2 Chromiums | | = 2 x | 51.996 | | = | | 103.992 |
| 9 Oxygens | | = 9 x | 15.9994 | | = | | 143.9946 |
| 4 Hydrogens | | = 4 x | 1.0079 | | = | | 4.0316 |
| | | | | | | 297.9978 |
Question: What is the percent Chromium in this salt?
| Mass of Chromium |
| Mass of the Salt |
X 100 = Percent Chromium
·
2 H2O
Moles/Mass
One of the most useful applications of the gram formula weight is the determination of the number of moles of a substance.
Question: How many moles of sodium dichromate dihydrate (Na2Cr2O7 ·
2 H2O) are present in 26.5 g of that material?
1 mole = 298.0 g therefore, = 0.0889 moles
Question: How many grams of acetone must I use if I want 3.6 moles?
Moles X g Formula Weight = Mass
3.6 moles X 58.08 g/mole = 210 g
Question: What is the volume of this much acetone?
Percent Yield
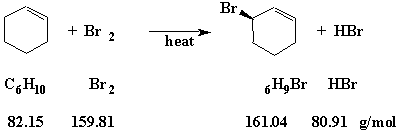
Before trying to calculate a percent yield, it is necessary to have a balanced equation. For the allylic substitution reaction of bromine (Br2) with cyclohexene (C6H10), the equation is
Question: In one experiment, we used 20.1 g of cyclohexene and 20.5 g of bromine. Which reagent is the "Limiting Reagent"; what is the "Theoretical Yield" of the product (3-bromocyclohexene); and, what is the "Percent Yield" if the reaction produced 5.2 g of product?
To determine the "Limiting Reagent" we first look at the balanced equation. That equation tells us that it takes one mole of bromine to react completely with one mole of cyclohexene. We must next calculate how many moles we have of each of the reagents.
Moles of Cyclohexene = = 0.245 moles
Moles of Bromine = = 0.128 moles
Since we have about twice as many moles of cyclohexene as bromine, we will run out of bromine first. Therefore, bromine is the "Limiting Reagent".
Since we have only 0.128 moles of the "Limiting Reagent" (Bromine), the maximum quantity of product that could be produced is only 0.128 moles. The "Theoretical Yield" of 3-bromocyclohexene is therefore
0.128 moles x 161.04 g/mole = 20.6 g (Significant Figures!)
The maximum amount of product that could be produced is 20.6 g
Question: But what is the "Percent Yield" if the reaction actually produced 5.2 g of product?
| Actual Yield |
| Theoretical Yield |