Experiment 3 - Extraction
Objective
In this experiment, you will separate the components of a commercial headache powder via an extractive process. This separation will be accomplished by taking advantage of the fact that each component contains different functional groups which will react differently when treated with a specified reagent.Introduction
Extraction is a widely used method for the separation of a substance from a mixture. It involves the removal of a component of a mixture by contact with a second phase. Solid-liquid and liquid-liquid extractions are commonly performed by batch and continuous processes. The removal of caffeine from coffee beans with dichloromethane is an example of a solid liquid extraction. Crystal violet may be removed from a water solution by liquid-liquid extraction with n-amyl alcohol (1-pentanol). Other common applications of liquid-liquid extractions involve:-
1Isolation of organic reaction products
-
2Removal of acid, base, and salt impurities
-
3Removal of organic acids and bases from other organic compounds
( 1 )
Kp =
| concentration of A in S (g/mL) |
| concentration of A in S' (g/mL) |
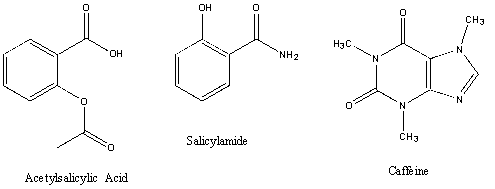
Figure 1
Separating Each Compound
Acetylsalicylic acid, Aspirin, is an organic acid; therefore, it is soluble in an organic solvent (diethyl ether), but will react with a basic reagent (:B) such as sodium hydroxide or sodium bicarbonate to produce the conjugate base of the acid. The conjugate base is a salt and is water soluble; therefore, it is removed from the organic solvent layer. Reacidification of this basic aqueous layer will regenerate the organic acid, which will precipitate from the aqueous solution due to the acid's limited solubility in water.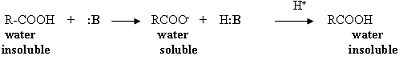
Figure 2
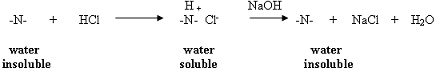
Figure 3
Figure 4
Pre-Lab
-
1Brief outline of procedure
-
2Reagent data (Aldrich/MSDS) (structure, formula weight, MP or BP, density (if applicable), Hazards) for salicylamide, caffeine, acetylsalicylic acid, diethyl ether, and ethanol
-
3Answers to any assigned questions

Figure 5
Questions
Question 1: Explain why diethyl ether is a superior solvent to hexane for extracting caffeine from an aqueous solution. The structure of caffeine may be found at the beginning of this experiment.
Question 2: When 100 mL of an aqueous solution containing 1.0 g of caffeine is extracted with 10 mL of chloroform at room temperature, 0.5 g of caffeine is transferred to the chloroform layer. Calculate the distribution coefficient of caffeine between chloroform and water at room temperature. Show all work.
Question 3: Write an equation showing the reaction of acetylsalicylic acid with sodium bicarbonate. What happens when this reaction mixture is treated with hydrochloric acid? (reaction equation would be appropriate)
Procedure
In a 50 mL Erlenmeyer flask, dissolve the contents of one packet of headache powder in 20 mL of diethyl ether. Since the binder, etc. are sometimes inert material, all of the powder may not dissolve, but this is not a problem. Pour the solution into the separatory funnel and use a fresh 5 mL of diethyl ether to transfer the remaining contents of the flask to the separatory funnel. Extract the ether solution with 20 mL of 3M HCl, then remove the aqueous layer (which is it, top or bottom???) into a beaker labeled "HCl layer". Remember, emulsions and the identification of the solute are common problems encountered in simple extractions. Emulsions sometimes are diminished by the addition of an ionic compound such as sodium chloride (NaCl) which changes the ionic strength of the aqueous layer and increases the solubility of some components. The determination of which layer is organic and which is aqueous is easily accomplished by knowing the densities of the solvents used. Also, one can add a drop of water to the seperatory funnel and observe whether the droplets dissolve in the upper layer, or pass through the lower.
Figure 6: Close-up of the distinction between layers
HCl Layer:
This solution contains the conjugate acid of caffeine. Since the majority of powders contain a small portion of caffeine, isolation will not be performed. The solution should be neutralized by the gradual addition of 6 M NaOH and discarded in the appropriate waste container.Bicarbonate Extract:
This solution contains the conjugate base of acetylsalicylic acid. Cool in an ice bath and carefully acidify this solution by the addition of 6 M HCl. Add the acid slowly since CO2 will be produced and effervescence will occur. The acetylsalicylic acid should begin to precipitate as the acid is added. Once the solution is acidic, collect the precipitate by vacuum filtration. The crude acetylsalicylic acid is (it needs to be) recrystallized as follows. Place the crude solid in a 25 mL Erlenmeyer flask and add 10 mL of hot water. Heat the mixture to boiling on the hot plate. Add just enough 95% ethanol to dissolve the solid (1 - 2 mL). Remove the flask from the hot plate and allow the contents to slowly cool to room temperature. Once the contents have reached room temperature, the flask may be placed in an ice bath to complete the process. The acetylsalicylic acid is isolated by vacuum filtration, dried, weighed, and a melting point is determined. (For more on determining a melting point, see pages 36-37).In-Lab Questions
Download and print the following worksheet. You will use this worksheet to record your answers to the In-Lab questions.Questions
Question 1: Amount of Acetylsalicylic acid in powder (see box) ___________________
Question 2: Amount of Acetylsalicylic acid recovered ___________________
Question 3: Percentage Recovery _______________
Question 4:
- Melting Point of Acetylsalicylic Acid _______________ (observed)
- Melting Point of Acetylsalicylic Acid _______________ (lit. reported)
Question 5: Amount of Salicylamide in powder ____________________
Question 6: Amount of Salicylamide recovered _____________________
Question 7: Percentage Recovery _______________
Question 8:
The two solids should be shown to your instructor and then placed in the appropriate waste container.
- Melting Point of Salicylamide _______________ (observed)
- Melting Point of Salicylamide _______________ (lit reported)