Lab 8 - Buffers
Purpose
To prepare buffers and measure the pH of each, and to prepare a buffer at a specific pH.Goals
-
1To learn to prepare buffers by both the direct and indirect methods.
-
2To learn to identify solutions that are buffers.
-
3To understand the how a buffer resists changes in pH upon addition of acid or base solutions.
Introduction
In dilute aqueous solutions, weak acids are slightly dissociated. They produce a small concentration of hydronium ion (H3O+) and an equal concentration of the conjugate base of the acid. Such dissociation reactions are equilibria, and equilibrium mathematics can be used to calculate concentrations of the species present in solution. Consider formic acid (CH2O2), which is what red ants inject when they bite. The acid dissociation constant Ka for formic acid is 1.7 x 10-4. The concentration of H3O+ present in a 0.010 M solution of formic acid can be calculated from the equilibrium expression and a reaction table.( 1 )
| HCOOH(aq) | + | H2O(l) | 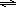 | H3O+(aq) | + | HCOO-(aq) | |
| initial | 0.010 | 0 | 0 | ||||
| Δ | -x | +x | +x | ||||
| equilibrium | 0.010 - x | x | x | ||||
( 2 )
Ka = 1.7 x 10−4 =
=
| [H3O+ ][HCOO− ] |
| [HCOOH] |
| x2 |
| 0.010 − x |
( 3 )
pH = −log[H3O+ ] = log(0.0012) = 2.92
( 4 )
HCOONa(s) + H2O(l) → Na+(aq) + HCOO−(aq)
( 5 )
pH = pKa + log
or pH = pKa + log
 |
| [base] |
| [acid] |
 |
 |
| moles of base |
| moles of acid |
 |
( 6 )
HA + H2O 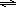 H3O+ + A-
H3O+ + A-
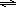 H3O+ + A-
H3O+ + A-( 7 )
Ka =
| [H3O+ ][A− ] |
| [HA] |
( 8 )
[H3O+] =
| Ka x [HA] |
| [A− ] |
( 9 )
pH = − log[H3O+ ] = − log Ka − log([HA]/[A−])
( 10 )
pH = pKa + log([A− ]/[HA])
( 11 )
| pH | = | pKa + log([A− ]/[HA]) |
| 4.00 | = | 3.77 + log([A− ]/[HA]) |
| [A− ]/[HA] = 1.70 |
Equipment
-
230 mL beakers
-
2100 mL volumetric flasks
-
110.0 mL volumetric pipet
-
1pipet bulb
-
150 mL graduated cylinder
-
110 mL graduated cylinder
-
150 mL beaker
-
1100 mL beaker
-
1glass stir rod
-
1MicroLab Interface
-
1MicroLab pH Measurement Instruction Sheet
-
1pH electrode in pH 7.00 buffer
-
1ring stand
-
1clamp
-
1250 mL beaker for electrode rinsings
-
1deionized water squirt bottle
-
1box Kimwipes
Reagents
-
~10 mL6.0 M acetic acid (HC2H3O2)
-
<10 gsolid sodium acetate trihydrate (NaC2H3O2 · 3 H2O)
-
~25 mL1.0 M NaOH
-
3 gsolid Na2HPO4 · 7 H2O
-
TBDsolid NaH2PO4 · H2O or solid Na3PO4 · 12 H2O
-
~15 mLpH 4.00 buffer
-
~15 mLpH 7.00 buffer
-
~15 mLpH 10.00 buffer
- 1.0 M NaOH in a dropper bottle for Part C
- 1.0 M HCl in a dropper bottle for Part C
Safety
Acetic acid, HCl and NaOH are corrosive. They can attack the skin and cause permanent damage to the eyes. If one of these solutions splashes into your eyes, use the eyewash immediately. Hold your eyes open and flush with water for at least 15 minutes. If contact with skin or clothing occurs, immediately rinse the affected area with water for at least 15 minutes. Have your lab partner notify your teaching assistant about the spill and your exposure. Students will have access to gloves due to the use of concentrated acetic acid during the lab period.Waste Disposal
All solutions can be disposed of down the sink drain followed by flushing with plenty of water.Prior to Class
Please read the following section of Lab Safety and Practices: Please read the following section of Lab Equipment: Please review the following videos: Please complete WebAssign prelab assignment. Check your WebAssign Account for due dates. Students who do not complete the WebAssign prelab are required to bring and hand in the prelab worksheet.Lab Procedure
Please print the worksheet for this lab. You will need this sheet to record your data. In this experiment, you will be using pH electrodes connected to the MicroLab Interface. pH electrodes have a thin glass bulb at the tip. They break easily and are costly to replace. Be careful not to shove the electrode into the bottom of a beaker or drop the electrode. There is a protective guard around the tip, which should remain in place at all times. The guard will not protect against careless treatment. Please use extreme care when using this equipment. Best results in using the electrodes are obtained if:-
•Electrodes are kept in standard pH 7 buffer solution when not in use.
-
•Immediately prior to use, the electrodes are rinsed with deionized water and gently blotted with a Kimwipe, then placed in the test solution.
-
•The electrodes are rinsed and blotted again after the measurement and returned to the pH 7 buffer solution.
Part A: Acetate Buffer by the Direct Method
1
Open the MicroLab program.
2
Make sure the pH electrode is connected to the interface.
3
Calibrate the pH electrode using the MicroLab instructions provided in the lab. Configure the MicroLab program to collect data as described in the instructions provided in the lab.
4
After the calibration and configuration are complete, measure the pH of each of the three buffer solutions of pH = 4.00 (red), pH = 7.00 (yellow), and pH = 10.00 (blue). Record the value in the digital display in WebAssign as a record of how accurately the probe is calibrated. Make sure the electrode is immersed in the solution and allow for a few seconds of equilibration.
5
Using the 10.0 mL volumetric pipet and a 100.0 mL volumetric flask, prepare 100.0 mL of 0.60 M HC2H3O2 by diluting the 6.0 M stock solution provided. (Hint: Remember your prelab exercise!) Be sure to condition your pipet before using it.
Question 1: Show your calculation for preparing the 0.60 M HC2H3O2 solution from your prelab assignment.
6
Using the other 100.0 mL volumetric flask, prepare 100.0 mL of 0.60 M sodium acetate solution by dissolving solid sodium acetate trihydrate (NaC2H3O2 · 3 H2O) in water and diluting to a total volume of 100.0 mL. (Hint: Remember your prelab exercise!)
Question 2: Show the calculation of how many grams of NaC2H3O2 · 3 H2O are needed from your prelab assignment.
7
Use a graduated cylinder to measure 30 mL of the 0.60 M acetic acid solution you prepared into a 50 mL beaker. Measure the pH of this solution and record it in Data Table A as solution 1A. Make sure the electrode bulb is fully immersed before measuring.
Table A: pH Data for Acetate Buffers (Direct Method)
8
Add 10 mL of your sodium acetate solution to the beaker containing acetic acid and stir with a clean stirring rod. Measure the pH of the solution and record it in Data Table A as solution 2A. After the measurement is complete, the solution may be discarded.
9
Use a graduated cylinder to measure 30 mL of the 0.60 M sodium acetate solution you prepared into a 50 mL beaker. Measure the pH of the solution and record it in Data Table A as solution 3A.
10
Add 10 mL of your acetic acid solution to the beaker containing sodium acetate and stir with a clean stirring rod. Measure the pH of the solution and record it in Data Table A as solution 4A. After the measurement is complete, the solution may be discarded.
11
Make solution 5A by mixing 20 mL of acetic acid solution with 20 mL of sodium acetate, stirring well. Measure its pH and record it in Data Table A as solution 5A.
Question 3: Explain the order of pH for the five solutions. Consider the relative amounts of acid and base in each.
Part B: Acetate Buffer by the Indirect Method
1
Place 30 mL of your 0.60 M acetic acid in a clean 100 mL beaker. Measure the pH of the solution and record it in Data Table B as solution 1B.
Table B: pH Data for Acetate Buffers (Indirect Method)
2
Determine whether or not this solution is a buffer solution, and enter your decision in Data Table B.
Question 4: How many mmol of acetic acid are present in your sample 1B? Show your work.
3
Add 4 mL of 1.0 M NaOH and mix the solution thoroughly. Measure the pH of the solution and record it in Data Table B as solution 2B.
Question 5: How many total mmol of NaOH have you added at this point? (Show your setup.) Enter this amount in Data Table B.
Question 6: Is this solution a buffer solution? Explain your reasoning. Fill in Data Table B with your choice.
4
Add in succession another 5 mL, then 6 mL and finally 10 mL of 1.0 M NaOH, mixing thoroughly and recording the pH after each addition.
5
Enter the cumulative number of millimoles (mmol) of NaOH added in each step in Data Table B. After the last addition, a total of 25 mL of NaOH should have been added.
6
Decide whether or not each solution is a buffer and enter your decision in Data Table B.
Part C: Phosphate Buffer by the Direct Method
1
Your lab instructor will assign you a target pH; write it in Data Table C. You need to generate 100 mL of the buffer solution starting with 3.00 g of Na2HPO4 · 7 H2O
2
Weigh out 3 g of Na2HPO4 · 7 H2O and record the exact mass in Data Table C. Transfer it carefully to a 100 mL volumetric flask. Add about 60 mL of deionized water, stopper the flask, and shake it to dissolve the solid. This will take a while.
Question 7: Will you need to use Na3PO4 · 12 H2O or NaH2PO4 · H2O in order to generate the desired pH? Explain your answer. Enter your choice in Data Table C.
Question 8: What mass of the other phosphate compound will you need to add in order to generate the desired pH? Show your work. Enter this amount in Data Table C.
3
Weigh out the calculated amount of the second phosphate compound and carefully add it to your volumetric flask. Stopper the flask and shake until all solid is dissolved. Then add deionized water up to the mark of your flask.
4
Pour some of the buffer solution into a beaker and measure its pH. Record the result in Data Table C.
Table C: Data for Phosphate Buffer
5
If the pH is different than the target pH, adjust it by adding 1.0 M HCl or 1.0 M NaOH dropwise until the desired pH is achieved. In your notebook, describe the action taken to adjust the pH of the buffer, e.g. "added 3 drops of 1.0 M HCl".
6
After your last measurement, stop and close the MicroLab software. Rinse all of your glassware with water, dry it and return it to the set-up area where you found it. Make sure the pH electrode is submerged in the pH 7 buffer solution.
7
Before leaving, enter your results in the InLab assignment. If all results are scored as correct, log out. If not all results are correct, try to find the error or consult with your teaching assistant. When all results are correct, note them and log out of WebAssign. The InLab assignment must be completed by the end of the lab period. If additional time is required, please consult with your teaching assistant.