Lab 1 - Thin Layer Chromatography
Objective
In this laboratory you will separate spinach pigments using thin layer chromatography (TLC).Introduction
Mixtures of compounds are very common in Organic Chemistry. Most reactions produce more than one product. Naturally occurring materials are only rarely 100% pure. It is therefore desirable to have a simple, fast and efficient way to determine the purity of Organic mixtures. The separation of a mixture by passing it, in solution, over an adsorbent (such as Alumina or Silica Gel) is the basic idea of Chromatography. Chromatography is a very general phenomenon. It involves the passage of a mobile phase across a stationary phase in a column. Usually a mixture of compounds is present in the mobile phase. As soon as the mixture comes in contact with the stationary phase, some or all of the components of the mixture are adsorbed on it. As additional mobile phase comes along, some or all of the mixture will dissolve and continue moving. This adsorption/solution process continues along the length of the column. If a proper choice of mobile phase, stationary phase, solvent and other operating parameters was made, the mixture will be separated in the column and its various components will emerge at different times. In Thin Layer Chromatography ("TLC"), a liquid solution is directly applied to a solid adsorbent. Capillary action draws a developing solvent up the TLC plate. As this solvent passes through the spot, the mixture will be dissolved and will begin to move with the solvent front. However, the adsorbent will also reabsorb part or all of the mixture. As more solvent comes by, the mixture will again go into solution, move further and be reabsorbed. Since different materials will be dissolved and reabsorbed at different rates, separation will take place. The slide is removed from the chamber once the solvent front reaches a predetermined spot near the edge farthest from the point of spotting. This passage of the solvent front through the adsorbent is known as developing the plate. The extent of separation, measured by retention factor ("Rf
") value differences, will depend on the relative solubilities and relative strengths of adsorption of the components of the mixture.
Organic compounds interact with absorbents by a variety of interactions. If the compound is non-polar, it can only have weak 'Van der Waals' attractions for the absorbent. However, more polar molecules may interact more strongly by a variety of mechanisms including dipole-dipole interactions, coordination, and hydrogen bonding. The most important rule of chromatography is that the more polar compounds will be absorbed most strongly on absorbents (stationary phases), while non-polar compounds will be only very weakly absorbed.
In a typical chromatography experiment, the non-polar compounds, since they are poorly absorbed, will be held least strongly and will move quickly through the plate. Polar compounds, on the other hand, will be slowed on their process through the plate by their strong interactions with the solid phase. This separation based on polarity will explain most of the chromatography encountered in this course.
Types of Adsorbents used in Chromatography
Listed in decreasing power of adsorption:- Alumina
- Activated Charcoal
- Magnesium Silicate
- Silica
- Starch
Solvents Commonly Used in Chromatography
Listed in decreasing polarity:- Acetic Acid
- Water
- Methanol
- Acetone
- Ehtyl acetate
- Diethyl ether
- Chloroform
- Methylene chloride
- Toluene
- Cyclohexane
- Petroleum ether
Rf
values will change when either of these factors is changed.
( 1 )
Rf =
| Distance spot traveled |
| Distance Solvent Traveled |
Pre-Lab
Answer all assigned WebAssign questions.Procedure
Caution:
Pentane is highly flammable. Perform only in a well ventilated hood.
Pentane is highly flammable. Perform only in a well ventilated hood.
1
We will start by extracting the pigments from the spinach juice: add 3 mL of spinach juice and 6 mL of pentane to a screw-capped test tube and shake vigorously for 1 minute.
2
Spin the mixture in a centrifuge for 5 minutes, after which time a transparent green top layer should be visible. You can watch a video that shows how to use our centrifuges.
3
Transfer the transparent green top layer using a Pasteur pipet to a clean 50-mL beaker.
4
Evaporate the pentane by heating the beaker on a hot plate at a low heat setting (about 95°C) for a few minutes until 1-2 mm of liquid remain. Remove from the hot plate immediately to prevent degrading the spinach pigments. If almost all of the solvent is accidentally evaporated, two or three drops of pentane may be added to redissolve the green residue. Adding a drop or two of pentane after evaporation will ensure better loading of the TLC plate.
5
Now you are ready to perform the thin layer chromatography separation. Please watch the video before you proceed.
6
Obtain a TLC plate and a developing chamber.
7
In the developing chamber, place about 5 mL of the TLC developing solvent (7:3 mixture of hexane:acetone). Be sure the depth of solvent is no more than 0.5 cm. If the start line should touch the solvent directly, the TLC experiment is ruined since some or all of the sample will be dissolved into the solvent pool.

Figure 1
8
Stopper the chamber to allow it to become saturated with solvent vapors. A cap is kept in place at all times, except when adding or removing a plate. Allow at least 5-10 minutes for the chamber to equilibrate before the first plate is developed.
9
Trace a light pencil line about 1 cm from the bottom of the plate and another light pencil line 5 cm up the plate from the first line. You should make your pencil marks on the papery side (not on the side with the glossy finish).
10
Use a microcapillary tube to load the extract onto the TLC plate. Allow the tip of the drop at the end of the capillary to just touch the plate. Blow lightly onto the plate after each drop is added to allow the solvent to evaporate. Each time you add sample to the spot, make sure it never gets any larger than it did the first time. This will ensure a very concentrated spot at the start line and will give the most concentrated spots (nearly round) on development of the plate.
11
After all spots have been applied, and all spots are dry, the plate may be placed into the developing chamber and capped immediately to avoid loss of the solvent saturated atmosphere. Almost immediately, the solvent will begin to migrate up the plate.
12
Once the solvent reaches the top line on the plate, remove it and allow the plate to dry.
13
As the plate dries, you will notice a change in its appearance. However, the spots will not be visible unless they are colored materials. The spots must be visualized. A UV lamp is the simplest way to visualize.
14
Place the dry plate on the bench top and allow the UV light to shine on it. If there is a spot, it will probably show as a different color of fluorescence than the background, or as a darkened area on the adsorbent.
Caution:
Never allow UV light to shine on anyone's eyes. Permanent eye damage may result. Be extra careful to keep the UV lamp pointed down at the bench top at all times.
Never allow UV light to shine on anyone's eyes. Permanent eye damage may result. Be extra careful to keep the UV lamp pointed down at the bench top at all times.
15
With a very sharp pencil or other sharp instrument, draw an outline of each spot in the adsorbent. Turn OFF the UV lamp and carefully put it away. Include your TLC plate with your lab worksheet.
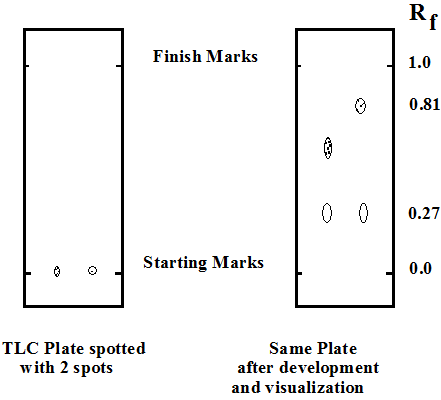
Figure 2
16
Calculate the retention factors for each one of the pigments on your plate.
In-Lab Questions
Please print the worksheet for this lab. You will need this sheet to record your data.Questions
1
How many pigments were you able to visualize and identify? List them and describe their appearance.
2
What is the Rf
value for each of the pigments in your TLC plate?
3
Were your Rf
values comparable to those obtained by other students in the class? If not, could you explain any observed discrepancies?
4
Which of the following pairs of compounds would be most easily separated by thin layer chromatography: n-octyl alcohol and 1-octene, hexadecane and octadecane, or chlorobenzene and bromobenzene? Justify your answer.
5
How could thin layer chromatography be used to aid in the identification of a compound?