Experiment 9 - Arenediazonium Salts
Objective
In this experiment, the use of one of the more specialized aromatic reactions, diazonium salts, is examined.Introduction
During the study of the chemistry of aromatic compounds, several reactions are encountered that allow for halogenation (Cl and Br only), nitration, alkylation, and acylation of the aromatic ring. Also, one is introduced to the influence of substituents on the ring, i.e., groups that are ortho-para directors, or meta directors. However, as one looks through the lecture text, it is apparent that various other substituents are seen in aromatic systems (OH, CN, I, etc.) and substitution patterns that do not fit the norm also are observed. These groups are usually added to the aromatic ring via more specific types of reactions. Arenediazonium salts are generated by the reaction of a primary amine with nitrous acid (produced from sodium nitrite) as shown below. The aromatic amines (anilines) are generated by the reduction of the corresponding nitro compound, which is easily prepared via electrophilic nitration of the ring (see nitration of methyl benzoate). The diazonium salts are unstable at temperatures above 5 - 10°C and some explode if allowed to dry. The aliphatic counterpart can be prepared in the same way; however, even at low temperature it is more unstable and can spontaneously decompose by loss of nitrogen to produce carbocation.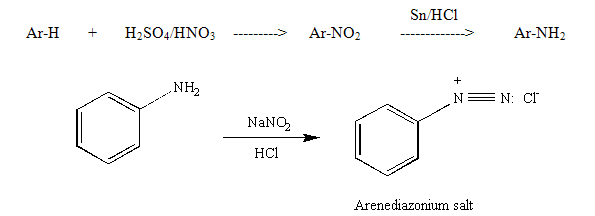
Figure 1
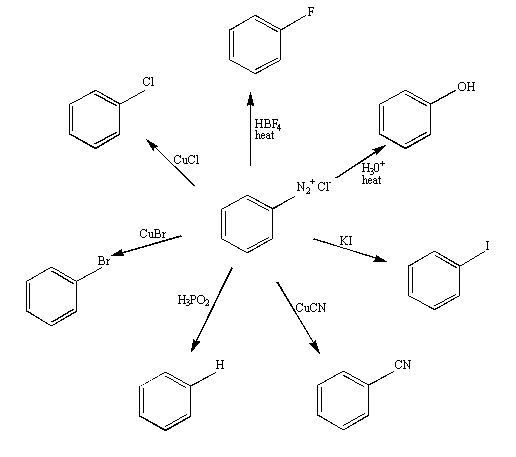
Figure 2
Figure 3
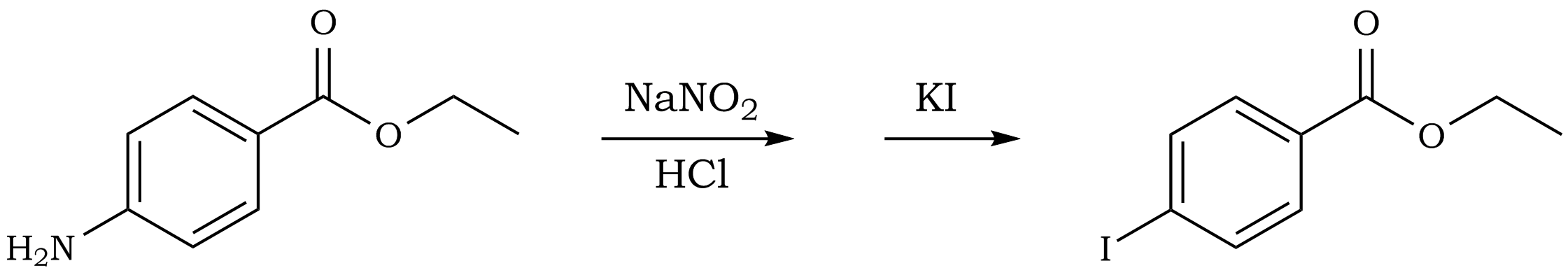
Figure 4
Pre-Lab
Complete the pre-lab assignment in WebAssign.Procedure
In a 50 mL Erlenmeyer flask, 0.5 g of ethyl 4-aminobenzoate (benzocaine) is dissolved in 8 mL of water containing 2 mL of 6 M HCl. Heating may be required for complete solution formation. The solution is cooled to 0 - 5°C in an ice bath and a solution of 0.23 g of sodium nitrite in 2 mL of water is slowly added. Keep the resulting solution cold and swirl the contents intermittently for a period of 5 - 10 minutes. Prepare a solution of 1 g of potassium iodide in 2 mL of water. Cool this solution in an ice bath then slowly add it, with swirling, to the cold solution of the diazonium salt. Allow the reaction mixture to slowly warm to room temperature (swirl occasionally) over a period of 10 - 15 minutes. Pour the aqueous reaction mixture into a separatory funnel and extract with 20 mL of diethyl ether. The aqueous layer is removed and the organic layer is washed successively (remove the aqueous layer each time) with 15 mL of 10% HCl, 15 mL of saturated sodium bicarbonate, and 15 mL of saturated sodium thiosulfate. Transfer the organic layer (yellow to orange in color) to a clean 50 mL Erlenmeyer flask and add a small amount of anhydrous sodium sulfate (cover the bottom of the flask) to dry the organic solution. Allow the solution to dry for 5 minutes, filter (or decant) the ether solution to remove the drying agent (you may use a small amount of ether, ~5 mL, to rinse the drying agent), and concentrate to yield the product. Prior to concentrating, check for purity of the product by TLC analysis using hexane:acetone, 7:3. Spot both the product solution and a solution of the starting material on the TLC plate and compare the Rf value of your product with that of the starting material. Determine the yield and percentage yield of the reaction. Perform an infrared analysis on your product and compare the spectrum to that of the starting material.In-Lab Questions
Download and print the following worksheet. You will use this worksheet to record your answers to the In-Lab questions.Questions
Record the following data.- Question 1: Amount of ethyl 4-aminobenzoate used _________________ g, _________________ mol
- Question 2: Amount of sodium nitrite used ___________________ g, ___________________ mol
- Question 3: Amount of potassium iodide used ___________________ g, ___________________ mol
- Question 4: Amount of ethyl 4-iodobenzoate produced _________________ g, _________________ mol
- Question 5: Theoretical yield of ethyl 4-iodobenzoate _________________ mol, _________________ g
- Question 6: Percentage Yield _______________________
- Question 7: Record your calculations.
- Question 8: What was the Rf value of the starting material? Of the product? Which one is the more polar?
- Question 9: You used infrared spectroscopy as a means to determine the absence of any starting material in the product. What infrared stretches were used?