| reference signals for 1H NMR | |||||||
|---|---|---|---|---|---|---|---|
| proton type | δ (ppm) | proton type | δ (ppm) | ||||
| reference | alkyl protons next to a functional group |
||||||
| Si(CH3)4 (TMS) | 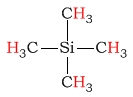 |
0.0 | C-H as part of an acetal/ketal | 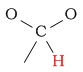 |
4.4 - 6.1 | ||
| hydrocarbons | C-H next to alcohol/ether | 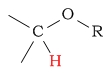 |
3.3 - 4.5 | ||||
| alkane protons | 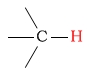 |
0.7 - 2.8 | C-H next to aldehyde/ketone | 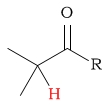 |
2.0 - 2.7 | ||
| alkane (methyl) protons | 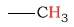 |
0.7 - 1.3 | C-H next to amine | 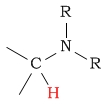 |
2.2 - 2.9 | ||
| alkane (methylene) protons | 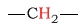 |
1.2 - 1.6 | C-H next to azide | 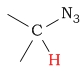 |
3.4 - 4.3 | ||
| alkane (methine) protons | 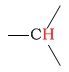 |
1.4 - 1.8 | C-H next to carboxylic acid | 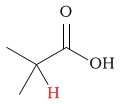 |
2.1 - 4.2 | ||
| aralkyl (benzylic) protons | 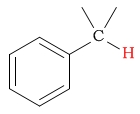 |
2.2 - 2.8 | C-H next to carboxylic acid halide | 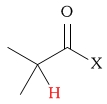 |
2.6 - 4.1 | ||
| alkene (allylic) protons | 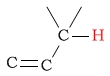 |
1.5 - 2.6 | C-H next to cyanate | 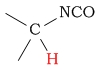 |
3.6 - 4.5 | ||
| alkene (vinylic) protons | 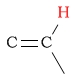 |
4.5 - 6.5 | C-H next to epoxide | 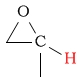 |
2.5 - 3.6 | ||
| alkyne (acetylenic) protons | 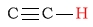 |
1.8 - 3.1 | C-H next to ester (carbonyl side) | 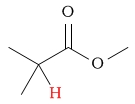 |
2.0 - 2.2 | ||
| arene (phenyl) protons | 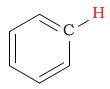 |
6.0 - 8.5 | C-H next to ester (ether side) | 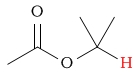 |
3.7 - 4.1 | ||
| protons that are part of a functional group |
C-H next to halide | 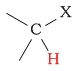 |
2.5 - 4.5 | ||||
| alcohol (alkyl alcohol) proton | 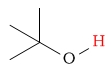 |
2.5 - 5 | C-H next to bromine | 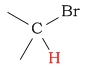 |
2.7 - 4.1 | ||
| alcohol (aryl alcohol/phenolic) proton | 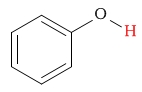 |
5 - 8 | C-H next to chlorine | 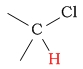 |
3.1 - 4.1 | ||
| aldehyde proton | 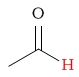 |
9.5 - 10.5 | C-H next to fluorine | 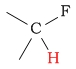 |
4.0 - 4.5 | ||
| amine proton | 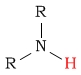 |
1 - 3 | C-H next to iodine | 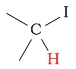 |
2.0 - 4.0 | ||
| carboxylic acid proton | 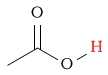 |
10 - 13 | C-H next to nitrile (cyanide) | 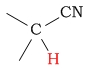 |
2.0 - 3.6 | ||
| C-H next to nitro | 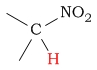 |
4.0 - 4.5 | |||||
| C-H next to sulfone | 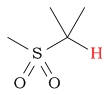 |
2.5 - 3.5 | |||||
| C-H next to thioalcohol/thioether | 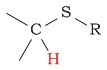 |
2.0 - 4.0 | |||||
| splitting patterns in 1H NMR | ||
|---|---|---|
| number of equivalent couplings |
multiplet appearance |
integral ratio |
| 0 | singlet (s) | 1 |
| 1 | doublet (d) | 1 : 1 |
| 2 | triplet (t) | 1 : 2 : 1 |
| 3 | quartet (q) | 1 : 3 : 3 : 1 |
| 4 | quintet | 1 : 4 : 6 : 4 : 1 |
| 5 | sextet | 1 : 5 : 10 : 10 : 5 : 1 |
| 6 | septet | 1 : 6 : 15 : 20 : 15 : 6 : 1 |
Note: "m" is frequently used to denote a higher-order multiplet; "br" is sometimes used to indicate a broad signal
| 3JHH coupling constants in 1H NMR | |||||
|---|---|---|---|---|---|
| cis-alkene | 4 - 12 Hz | cis-cycloalkane (Jax,eq) | 4 - 5 Hz | ||
| trans-alkene | 12 - 18 Hz | trans-cycloalkane (Jax,ax) | 10 - 14 Hz | ||
| reference signals for 13C NMR | |||||||
|---|---|---|---|---|---|---|---|
| carbon type | δ (ppm) | carbon type | δ (ppm) | ||||
| hydrocarbons | next to a functional group | ||||||
| methyl | 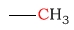 |
0 - 35 | C next to alcohol | 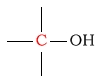 |
50 - 65 | ||
| methylene | 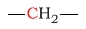 |
15 - 40 | C next to amine | 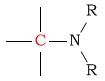 |
35 - 50 | ||
| methine | 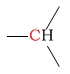 |
25 - 50 | C next to azide | 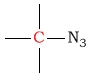 |
10 - 60 | ||
| quaternary carbon | 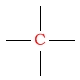 |
30 - 40 | C next to bromine | 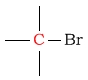 |
20 - 40 | ||
| alkene | 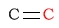 |
100 - 150 | C next to chlorine | 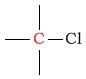 |
25 - 50 | ||
| alkyne | 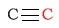 |
65 - 90 | C next to ether | 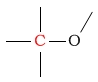 |
50 - 75 | ||
| arene | 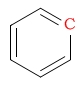 |
110 - 175 | C next to fluorine | 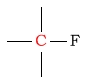 |
80 - 95 | ||
| in a functional group | C next to iodine | 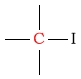 |
0 - 20 | ||||
| C in an aldehyde/ketone | 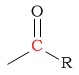 |
190 - 220 | C next to a nitro | 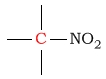 |
65 - 80 | ||
| C in an amide | 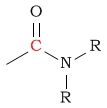 |
150 - 180 | C next to thioether | 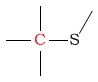 |
20 - 45 | ||
| C in a carboxylic acid or ester | 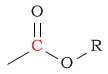 |
160 - 185 | |||||
| C in a cyanate | 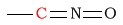 |
120 - 130 | |||||
| C in a nitrile (cyanide) | 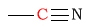 |
110 - 125 | |||||