Measurements – Density of a Cylinder
Objective
The purpose of this laboratory exercise is to determine the density of the material of the cylinder by measuring the mass, length, and diameter of a cylinder. You will also learn to use various data collection and analysis methods, which will aid in future lab activities.Equipment
- Numbered cylinder
- Vernier calipers
Introduction
You will practice taking some dimensional measurements and use them to determine the density of a cylinder made of an unknown material. As a reminder, this is the equation for the volume of a cylinder. In this equation, r is the radius of the cylinder and L is its length. (This is easy to remember if you think about slicing the cylinder into circular slices. Each slice has areaπr2
and the slices run all along the length L.)
For any object, its density ρ
is defined as
the ratio of the object's mass to its volume.
Procedure
Please print the worksheet for this lab. You will need this sheet to record your data.Density of a Cylinder
1
Make note of the number written on the end of your cylinder. Also make note of the cylinder's mass, which is written on the other end.
2
Measure the length of the cylinder with the Vernier calipers. See Measuring with Vernier Calipers if you are not sure how to read the calipers.
3
It is common practice to attempt to obtain a more accurate value of a measured quantity by making many measurements and then averaging them together. You will do this with the diameter of the cylinder to obtain a better value. Measure the diameter at a number of pleaces along the cylinder's length, and average.
4
Finally, find the cylinder's density. Compare it to the Densities table below to figure out what material it might be.
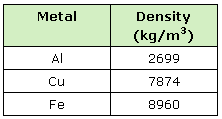
Table: Densities