How Do We Get Light from Matter: The Chemistry of Fireworks
Organization
-
•Mode: Inquiry, Groups of 3
-
•Grading: Lab Performance, Lab Notebook, Post-Lab Report
-
•Safety: Goggles, Lab coat, Long Hair Pulled Back
Goal:
In this lab you will explore the chemistry behind fireworks displays. You will heat different chemicals in a Bunsen burner and observe the light emitted by those chemicals. You will reach conclusions about the nature of the emitted light and be able to explain to someone the chemistry behind the visual effects seen during a fireworks display.
In this lab you will explore the chemistry behind fireworks displays. You will heat different chemicals in a Bunsen burner and observe the light emitted by those chemicals. You will reach conclusions about the nature of the emitted light and be able to explain to someone the chemistry behind the visual effects seen during a fireworks display.
| CHEM 115 Expt. 1 | Chemical Classification | Possibility Of: | NFPA Codes | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
How Do We Get
Light from Matter? |
Poison A
|
Flammable Gas
|
Flammable Liquid
|
Combustable Liquid
|
Reacts with Water
|
Oxidizer
|
Organic Peroxide
|
Poison B
|
Corrosive Acid
|
Corrosive Base
|
Irritating or Harmful
|
Misc. Hazard
|
No Hazard
|
Fire
|
Sudden Release of Pressure
|
Reactive
|
Immediate (Acute) Health Hazard
|
Delayed (Chronic) Health Hazard
|
Fire
|
Health
|
Reactivity
|
Special Precautions
|
| Barium Nitrate | X | X | 0 | 1 | 1 | |||||||||||||||||
| Strontium Nitrate | X | X | X | X | 1 | 1 | 1 | |||||||||||||||
| Sodium Nitrate | X | X | 0 | 2 | 0 | |||||||||||||||||
| Magnesium Metal, Powder | X | X | X | 1 | 3 | 0 | ||||||||||||||||
| Iron Metal, 20 Mesh | X | X | 1 | 2 | 0 | |||||||||||||||||
I: Background
Fireworks make use of combustion reactions to propel the device and to excite the chemicals that produce the characteristic light and sound effects. Combustion reactions require a fuel source and an oxidant, and usually need a spark to get started. Fuel sources are generally carbon-rich matter, like gasoline, wood, or charcoal, but they can also be elements like magnesium or hydrogen. Oxidants are typically oxygen-rich compounds. Oxygen gas from the atmosphere is the oxidant used in a car engine and a charcoal grill, but other chemical oxidants are employed (most commonly perchlorate, chlorate, and nitrate salts). A spark, flame, or heat is generally needed to kick-start a combustion reaction—often the fuel can be stored in the presence of oxidants for long periods of time safely, as long as heat and flame are kept away. Combustion reactions are characterized by the generation of lots of heat and usually lots of gas.The Anatomy of a Firework
A schematic of a firework is shown below (Figure 1).See www.howstuffworks.com/fireworks for a detailed description of fireworks.1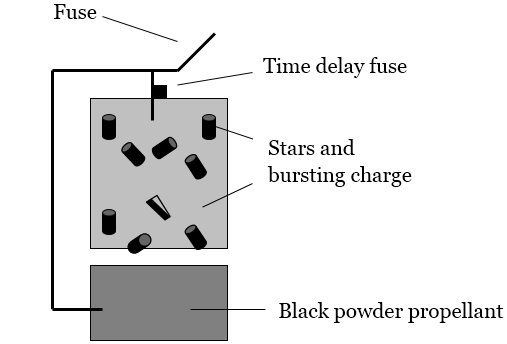
Figure 1: Schematic of a Firework
-
•Black Powder: Generally recognized as invented by the Chinese more than 1,000 years ago,The Chinese, Hindus, Greeks, Arabs, English and Germans have all claimed the honor of discovering black powder. There are early accounts of Chinese firecrackers, Roman Candles and Greek Fire. Roger Bacon (around 1242) of England and Berthold Schwartz (around 1300) of Germany left written records of their experiments. In their documents, they proved that they had identified the explosive property of sulfur, carbon, and potassium nitrate. Bacon published a formula for Black Powder; Schwartz invented the gun around 1300 which resulted in further refinement of black powder.2 little improvement has been made in the formulation of this combustion-ready powder, consisting of potassium nitrate (the oxidizing agent), charcoal, and sulfur (the fuels). A little spark is all it takes to get this oxidation-reduction reaction going.
-
•Stars: Stars are responsible for the spectacular color effects seen during a fireworks display. Each star consists of a fuel, an oxidizing agent, a coloring agent, and an inert binding material to hold the star together. They are little pellets of combustion chemistry waiting for the spark to get them going. While the coloring agents are initially solid compounds, the heat of the combustion reaction converts these compounds into their atomic components and vaporizes them into the gas phase.
-
•Flashes and Sparkles: Other chemicals are used to produce the white flashes and gold and silver sparkles seen in fireworks displays.
-
•Booms and Whistles: The rapid conversion of the reactants into gaseous products during the combustion reaction in a firework can create loud noises—if the gases expand at a speed above the speed of sound, you'll hear a boom. If the gases are released through a narrow opening, you'll hear a whistle.
II: Exercises
You'll be observing your flame by using your eye as a visible light detector and with an Ocean Optics spectrophotometer, which uses a fiber optic cable and a visible light detector. Use of the EquipmentOcean Optics Spectrophotometer:
The instructions are near the computers. One person aims the cable, the other operates the computer.
The instructions are near the computers. One person aims the cable, the other operates the computer.
1
Make sure the cap is removed from the end of the fiber optic cable (the flexible, metal-wrapped cable).
2
Make sure the computer is on (select the "classes" login if needed) and the Spectra Suite software is launched.
3
Aim the fiber optic cable at the source of light.
4
Start collecting the spectrum by either pushing the F3 button on the keyboard, or by moving the mouse to the green triangle "Start" button.
5
Move the fiber optic cable closer or further away to increase or decrease the intensity of the spectrum as needed.
6
When the spectrum looks good, push the F3 button on the keyboard or move the mouse to the "Stop" button to freeze the spectrum on the screen.
7
Click on the spectrum to put a cursor on the screen. Move the cursor by moving the mouse and clicking the new position. The value of the wavelength is displayed in a box to the left and below the spectrum.
8
If you want to collect a different spectrum, push the F3 button again—this replaces the previous spectrum.
9
To add another spectrum to the same graph, click on the spectrum icon, hit "Accept", and push F3—note, this function can be a bit tricky when storing the next spectrum.
10
When in doubt, if you need to clear the spectrum or spectra, just exit the software and open it again.
Part A: Flame Tests
Caution:
-
•READ THROUGH this section BEFORE lighting the flame!
-
•Do not leave a burning Bunsen burner unattended—make sure the gas is off anytime it is not in use.
-
•Long hair must be tied back.
Use of the Bunsen Burner:
In a Bunsen burner, propane gas is the fuel, and the oxidant is oxygen from air in the room. You can adjust the fuel/oxidant ratio being burned in your Bunsen burner in a couple of ways, depending on your Bunsen burner. Near the base of all Bunsen burners, you should see some holes through the metal casing of the burner tube. This is where the oxidant gets into the fuel mixture for burning. To adjust the fuel/oxidant ratio, you can adjust the size of these holes. For some Bunsen burners, the tube screws onto the base, and you can adjust the size of the holes by screwing or unscrewing the tube. Other Bunsen burner tubes are permanently attached to the base, but there is a metal grate with holes in it that you can twist around the burner to adjust the size of the holes. The propane is provided by a valve on the utility lines running along the bench.
In a Bunsen burner, propane gas is the fuel, and the oxidant is oxygen from air in the room. You can adjust the fuel/oxidant ratio being burned in your Bunsen burner in a couple of ways, depending on your Bunsen burner. Near the base of all Bunsen burners, you should see some holes through the metal casing of the burner tube. This is where the oxidant gets into the fuel mixture for burning. To adjust the fuel/oxidant ratio, you can adjust the size of these holes. For some Bunsen burners, the tube screws onto the base, and you can adjust the size of the holes by screwing or unscrewing the tube. Other Bunsen burner tubes are permanently attached to the base, but there is a metal grate with holes in it that you can twist around the burner to adjust the size of the holes. The propane is provided by a valve on the utility lines running along the bench.
-
•A: the flame
-
•B: distilled water
-
•C-1 and C-2: barium nitrate, Ba(NO3)2, solution and solid
-
•D-1 and D-2: strontium nitrate, Sr(NO3)2, solution and solid
-
•E-1 and E-2: sodium nitrate, NaNO3, solution and solid
-
•F: iron metal
-
•G: magnesium metal
-
•the appearance of the flame before you put anything into it
-
•the appearance of each chemical before you put it in the flame
-
•what you observe when you put the chemical in the flame (this is the flame test)
-
•a sketch of the spectrum, noting the wavelength of the peak
-
•a conclusion about whether the light emitted is continuous or a line emission
Instructions for Flame Tests
One person will perform the flame test, another will aim the cable at the flame, and the third operates the computer to collect the spectrum.1
Rinse off all spatulas at the distilled water sink, and dry them.
2
Place samples in a series of small test tubes. Fill another test tube with distilled (not tap) water. Record which sample you are testing.
Caution:
Long hair must be tied back. Goggles must be worn. Things will get hot, so be careful! Have a beaker of water on hand to put out any burning cotton swabs. Keep the area around the Bunsen burner cleared of papers, books, and other flammable materials.
Long hair must be tied back. Goggles must be worn. Things will get hot, so be careful! Have a beaker of water on hand to put out any burning cotton swabs. Keep the area around the Bunsen burner cleared of papers, books, and other flammable materials.
3
Turn the gas on, and light the flame with the striker. Adjust the gas valve on the bench to get a flame that is about 3 inches tall. Adjust the valve or grating at the base of the Bunsen burner to obtain a hot flame, with a blue cone inside the main flame. If you have a very yellow, smoky flame, the amount of air in the fuel/air mixture needs to be increased by opening the valve or grating at the base of the Bunsen burner. Be cautious when adjusting the flame. Once the flame is adjusted, you may proceed. However, if you leave the bench, turn the Bunsen burner off.
4
Sample Application
-
a.Solutions: Put a spatula in the sample and then in the flame. Wait a few seconds for the solution on the spatula to vaporize. Do a few trials, letting everyone see the color of the flame, until you figure out the best way to get the strongest flame.
-
b.Solids: Dip a cotton swab in the solid, then place the swab in the flame. Be sure to test a swab without sample present.
5
When you are ready, collect the spectrum.
6
Turn the gas off while evaluating the spectrum.
7
Repeat with the next test tube of sample using a clean spatula or a new swab.
8
For each sample, note the color of the flame, sketch the spectrum, measure the width of the peak responsible for the effect seen, and the wavelength of the peak.
Part B: Designing and Observing Your Own Firework Star
1
For this experiment, use solid Ba(NO3)2, Sr(NO3)2, and NaNO3, along with solid Fe and Mg. In a clean test tube, using a clean spatula for each ingredient, add together one small spatula—full of whichever combination of compounds you wish—to investigate. Cover the top of the test tube with a piece of paper and invert to mix the ingredients.
2
Turn on your Bunsen burner. Dip a clean swab into your test sample, and record the effects you see.
3
Write a description of the firework "star" that you designed in your laboratory notebook.
Clean Up:
Turn off your Bunsen burner, invert it, and tap it gently to knock out any residual chemicals. Wipe all solids on the bench into a paper towel, and shake the chemicals into the solid waste container in the hood, along with any solids from the test tubes. All solutions should be poured into the hazardous waste container for liquids in the hood. The paper towel and splints can be thrown into the trash can.
Turn off your Bunsen burner, invert it, and tap it gently to knock out any residual chemicals. Wipe all solids on the bench into a paper towel, and shake the chemicals into the solid waste container in the hood, along with any solids from the test tubes. All solutions should be poured into the hazardous waste container for liquids in the hood. The paper towel and splints can be thrown into the trash can.
