Lab Investigation 1 - Why Do Liquids Evaporate at Different Rates?
Guiding Question
Why do liquids evaporate at different rates?Introduction
Have you ever had a sunburn or high fever? If so, you may have used rubbing alcohol to help cool your skin. The alcohol works because it evaporates quickly and lowers skin temperature. The evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. The magnitude of temperature decrease is related to the strength of intermolecular forces of attraction. Rate of evaporation can be described as the change in temperature divided by the time it takes to reach the lowest temperatureΔT/Δt (°C/s).
Goals
As you complete this investigation you will:-
1Use a molecular model kit to build a model to describe the attractive forces between these molecules.
-
2Devise a method to measure the evaporation rates of seven colorless liquids.
-
3Develop an explanation for the differences in evaporation rates of these liquids.
Materials Available for Use
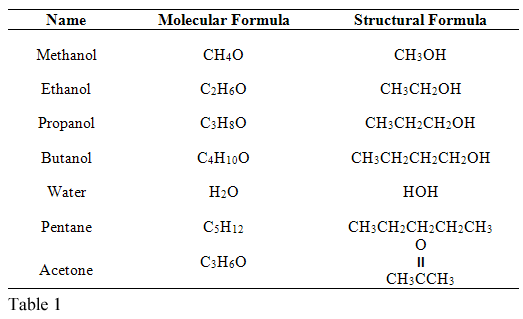
- Distilled water, H2O
- Acetone, C3H6O
- Methanol, CH4O
- Pentane, C5H12
- Propanol, C3H8O
- Ethanol, C2H6O
- Butanol, C4H10O
- Rubber bands
- Chromatography paper
- Molecular model kit
- Vernier LabPro system with temperature probe
Safety Precautions
Caution:
Wear goggles at all times.
Wear goggles at all times.
Getting Started
Use the Molecular Model kit to make models of each of the compounds that will be studied in Investigation 1 and consider the following questions as well as those in the PreLab activity.-
•What are the shapes of the molecules?
-
•Which molecules are polar and which are nonpolar?
-
•Which molecule has the strongest intermolecular forces?
Procedure
1
Set up the data collection program to collect data for 300 seconds.
2
Wrap the probe with chromatography paper and secure with a rubber band. Wrapped probes provide more uniform liquid amounts, and generally greater ΔT
values than bare probes.
3
Stand the probe in the liquid until the temperature reading is stable, hit start and collect data for 15 seconds to establish the initial temperature, Ti. Then remove the probe from the liquid.
4
Use the statistics function to find the minimum and the maximum temperature readings.
NOTE: The minimum temperature reading may occur before 300 seconds for some liquids.
Report
Once you have completed your research, you will need to prepare an investigation report that consists of three sections. This report may require more than 2 pages with data tables. This report must be typed and any diagrams, figures, or tables should be embedded into the document.- Section 1: What concept were you investigating, and how does it relate to the guiding question? Why do liquids evaporate at different rates? Describe and compare the relevant types of intermolecular forces and how they would impact evaporation rates.
- Section 2: How did you go about your work and why did you conduct your investigation in this way? Specifically, why measure temperature to find the rate of evaporation?
- Section 3: What is your argument? Consider what molecular variables you think affect the evaporation rates of liquids? Relate your data to intermolecular forces. Try graphing evaporation rate vs. molecular weight for the alcohol series to visualize your conclusion. Compare the evaporation rate of molecules with similar molecular structures. Use all your data.
- This third section is where you not only present your data, but use the values you obtain as evidence in your reasoning. Statements like, "see data table for values" are not acceptable!
Remember: An argument is not just an answer to the question. It is a claim or conclusion supported by evidence with a rationale for why the evidence supports the claim or conclusion.