Lab Investigation 3 - What are the Unknown Metals? Which Metals Make the Best Battery?
Guiding Question
Which two metals with their corresponding electrolyte solutions will make the highest voltage battery?Introduction
Oxidation-reduction reactions involve a transfer of electrons. In a spontaneous redox reaction, electrons flow from the oxidizing reactant (reducing agent) to the reducing reactant (oxidizing agent). If the two half-reactions can be separated, this flow of electrons, instead of occurring at the surface of the metal, occurs through an external wire and an electric current is generated. This is called a voltaic cell (or galvanic) and is exactly how a battery works. A battery, like the ones found in a flashlight or calculator, contains oxidizing and reducing substances. As the electrons are transferred, they are "tapped" in order to provide the voltage necessary to power the flashlight or calculator. In order for a redox reaction to serve as a source of power, the reaction must be spontaneous. Metal ions and metals exhibit characteristic values of reduction potentials. These values are of interest because they can be used to predict possible oxidation-reduction reactions between metals and their ions. This information can also be used to design batteries made up of two metals and their ions. Your group will use data collected from various electrochemical cells to predict the identities of four metals. Then you will predict and verify the best battery that can be made from a combination of two metals and their corresponding electrolyte solutions.Goals
As you complete this investigation you will:-
1Develop a procedure for measuring voltage developed by galvanic cells constructed from combinations of five metals and their corresponding electrolyte solutions.
-
2Determine the reduction potentials and identities of four of the metals.
-
3Outline a battery design using two of the five metals and their corresponding electrolyte solutions that will give the largest voltage possible with these materials.
Materials Available For Use
- Copper (Cu)
- 1.0 M CuSO4(aq)
- 4 metals identified only as M1 through M4
- 4 metal ion solutions (1.0 M) identified as M1 through M4, matching the metals
- 1.0 M NaNO3(aq)
- Small beakers
- Chromatography paper
- Vernier LabPro system with voltage probe
- Table of Standard Electrode Potentials
Safety Precautions
Caution:
Wear goggles at all times.
Wear goggles at all times.
Caution:
Dispose of any excess solutions in appropriate containers. Several are heavy metals that should not go down the drain.
Dispose of any excess solutions in appropriate containers. Several are heavy metals that should not go down the drain.
Caution:
Solution M4 will stain your skin and clothes. Wear gloves.
Solution M4 will stain your skin and clothes. Wear gloves.
Getting Started
In this laboratory you will construct and evaluate several voltaic cells. These cells will consist of two half-cells connected via a salt bridge. When the circuit is complete, electrons will flow spontaneously from the anode, where oxidation is occurring, to the cathode, where reduction is occurring. The potential, or EMF, of a cell is a measure of the tendency of the electrons to flow, and is dependent on the difference in oxidizing or reducing strength of the two half-cells. Since one half-cell reaction is known, the other can be calculated using Equation 1.( 1 )
E °cell = E °red + E °ox
-
•How will you use the voltage data to determine the identity of the unknown metal?
-
•Once you have identified all the metals, what experiment could you do as a check?
-
•What other (physical) properties of the metals might help you distinguish one from the other? Your instructor must approve additional experiments you plan to carry out.
Solution Preparation
You will be given copper and 4 unknown metals identified only as M1 through M4. You will need ion solutions matching the metals. Each table should prepare 100 mL of 1.0 M salt solution for each of the salts labeled M1 through M3 (M4 will be prepared for you). The molar mass of each salt is on the label. Prior to lab read over the section on using volumetric glassware.Procedure
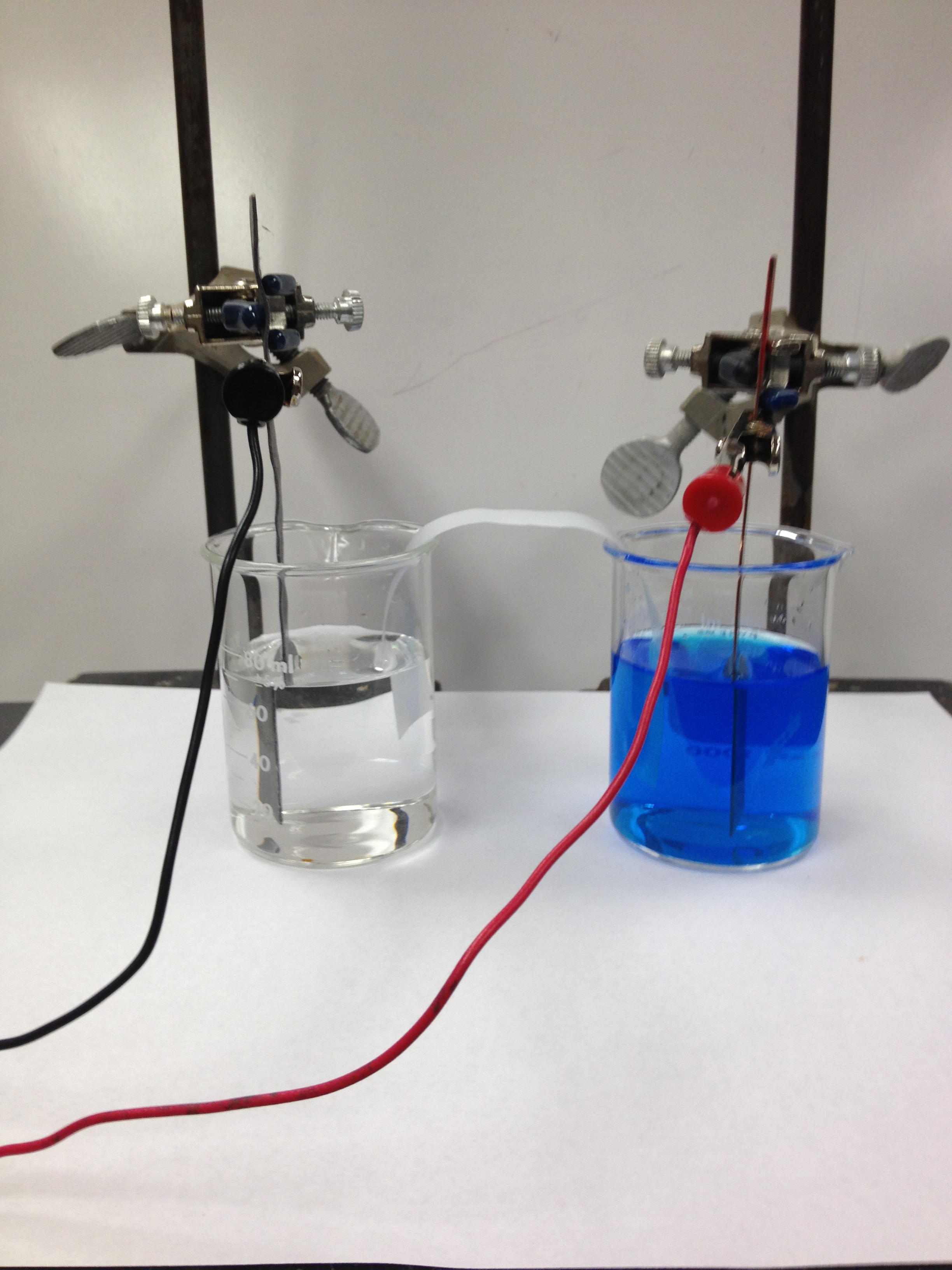
Figure 1
-
1Be sure to clean the surface of the metals with steel wool before use.
-
2Use the strips of chromatography paper soaked in NaNO3 for a salt bridge.
-
3The red lead is attached to the positive electrode, the cathode of a voltaic cell, and reduction occurs at the cathode. The black lead is attached to the negative electrode, the anode of a voltaic cell.
-
4Assume copper is being reduced.
Interactive Poster Session
Once your group has completed your work, prepare a whiteboard that you can use to share and justify your ideas. See the handout provided for details on this process.Report
Once you have completed your research, you will need to prepare an investigation report that consists of three sections. This report may require more than 2 pages with data tables. This report must be typed and any diagrams, figures, or tables should be embedded into the document.- Section 1: What concept were you investigating, and how does it relate to the guiding question? What are the unknown metals? Which metals make the best battery? Include in your discussion electrochemistry, oxidation-reduction reactions, voltaic cells, and batteries.
- Section 2: How did you go about your work and why did you conduct your investigation in this way?
- Section 3: There is an argument for the identity of the metals and for the choice of metals for the battery. You should include a data table with the voltage for each cell and the identity of the unknown metal. You must justify your identification of the metals by comparison with standard reduction potentials. Be sure to justify your choice of materials for the battery, which can be ANY two metals that you identified, not just the systems you used for identification.
- This third section is where you not only present your data, but use the values you obtain as evidence in your reasoning. Statements like, "see data table for values" are not acceptable!
Remember: An argument is not just an answer to the question. It is a claim or conclusion supported by evidence with a rationale for why the evidence supports the claim or conclusion.