Lab 12 - Rate Properties of an Iodide Oxidation Reaction
Goal and Overview
The rate law for the reduction reaction of peroxodisulfate (PODS) by iodide:S2O82−(aq) + 2 I−(aq) → I2(aq) + 2 SO42−(aq)
will be determined. The orders of reaction with respect to PODS and to iodide will be found by measuring rates for various
concentrations of the reactants. Rates will also be measured at three additional temperatures, such that the Arrhenius equation can be used to calculate the activation energy and the pre-exponential factor for the reaction.
Objectives and Science Skills
-
•Understand and explain common factors that influence the rates of chemical reactions.
-
•Apply linear fitting methods to find relationships between dependent and independent variables.
-
•Quantitatively and qualitatively evaluate experimental data to determine the reaction orders, rate constant, Arrhenius factor, and activation energy of an iodine clock reaction.
-
•Identify and discuss factors or effects that may contribute to deviations between theoretical and experimental results and formulate optimization strategies.
Suggested review and external reading
-
•relevant reference and textbook information on kinetics and dilutions
Background
Thermodynamics describes the equilibrium phases and concentrations of reactants and products under a given set of conditions. It gives no information on how quickly the equilibrium concentrations are reached. Chemical kinetics is the study of chemical reaction rates. Understanding how fast a reaction occurs, along with the factors that affect the rate, often helps to determine exactly how a reaction is occurring (the mechanism of the reaction). The speed with which a reaction occurs is described by the reaction rate. For a simple reaction likea + b → c + d,
the reaction rate can be expressed as concentration change with respect to change in time. Since the stoichiometry of the reaction above involves coefficients of 1 only, there is a simple relationship between the rates of formation of each product and the rates of consumption of each reactant. The negative signs on the reactant terms ensure that the calculated rate is positive for the forward reaction (the reactant concentration change is negative when the reaction proceeds from left to right).
( 1 )
Rate =
=
=
= −
= −
| change in concentration |
| time interval |
| Δ[c] |
| Δt |
| Δ[d] |
| Δt |
| Δ[a] |
| Δt |
| Δ[b] |
| Δt |
( 2 )
Rate ∝ [a]m[b]n or Rate = k[a]m[b]n
( 3 )
k = Ae−Ea/RT so Rate = Ae−Ea/RT[a]m[b]n
The Peroxodisulfate Iodide Clock Reaction (PODS/I– reaction)
In this reaction, two iodide ions provide one electron each to reduce the peroxodisulfate ion, S2O82–, to form two stable sulfate ions and molecular iodine. The overall redox reaction is shown below.( 4 )
S2O82−(aq) + 2 I−(aq) → I2(aq) + 2 SO42−(aq)
( 5 )
Rate = −
= −
=
=
= k[S2O82−]m[I−]n = Ae−Ea/RT[S2O82−]m[I−]n
| Δ[S2O82−] |
| Δt |
| Δ[I−] |
| 2Δt |
| Δ[I2] |
| Δt |
| Δ[SO42−] |
| 2Δt |
( 6 )
I2(aq) + 2 S2O32−(aq) → S4O62−(aq) + 2 I−(aq)
[S2O32−]
goes to zero, the I2 produced will no longer be removed and the solution turns blue. By using a known quantity of S2O32–, the rate can be calculated in terms of [S2O32−].
( 7 )
Rate =
= −
=
= k[S2O82−]m[I−]n
| Δ[I2] |
| Δt |
| Δ[S2O32−] |
| 2Δt |
| [S2O32−]initial |
| 2t |
Rate =
= −
=
= k[S2O82−]m[I−]n
| Δ[I2] |
| Δt |
| Δ[S2O32−] |
| 2Δt |
| [S2O32−]initial |
| 2t |
-
•To determine m, use different concentrations of PODS.
-
•To determine n, use different concentrations of I–.
-
•Once you have found m and n, determine the average value of the rate constant k at room temperature.
Notes:
-
•Bring stop watches or digital watches for recording reaction times in seconds.
-
•You will do this experiment with a partner – one person will mix while the other times.
-
•Half of the pairs will do Part 1a and half will do Part 1b (unless otherwise instructed).
-
•Every pair will do Part 2 (unless otherwise instructed).
-
•Every pair will put their times on the board so that the entire class will have good data.
-
•Before starting Part 1, set up the solutions for Part 2 (but do not mix them). The solutions need time to reach the temperature of their water bath.
Procedure
Note: Wash your glassware before you use it, and return it clean. Dedicate one pipet to each solution (don't mix and match).
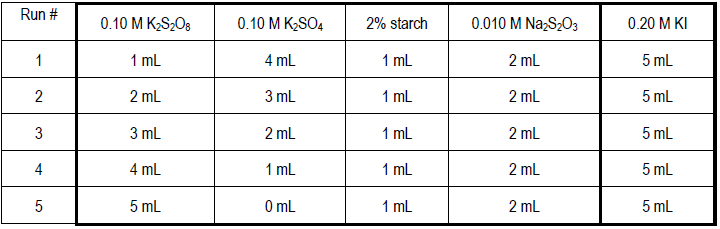
Part 1a: Reaction Order with Respect to Peroxodisulfate Ion (PODS), m
There are five runs in this series. The following table gives the volumes of reagents that must be accurately pipetted into a clean, dry 150-mL beaker for each trial.1
Prepare the timepiece.
2
Obtain 5 mL of 0.20 M KI solution in a volumetric pipet.
3
Start to record the time halfway through the addition of the KI.
4
You may want to record the draining time to get an approximate error in reaction times.
5
Gently swirl the beaker to keep the solution mixed.
6
Record the time of the first appearance of the dark blue color in seconds.
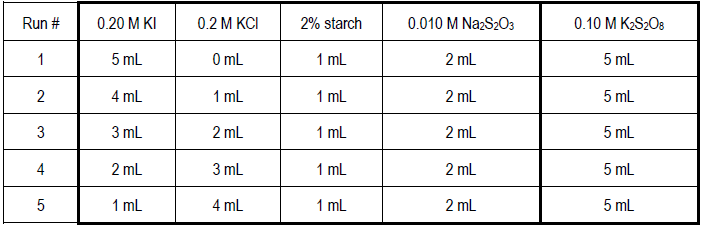
Part 1b: Reaction Order with Respect to Iodide Ion, n
There are five runs in this series. The following table gives the volumes of reagents that must be accurately pipetted into a clean, dry 150-mL beaker for each trial.1
Prepare the timepiece.
2
Obtain 5 mL of 0.10 M K2S2O8 solution in a volumetric pipet.
3
Start to record the time half way into the addition of the 5 ml of K2S2O8.
4
You may want to record the draining time to get an approximate error in recorded reaction times.
5
Gently swirl the beaker to keep the solution mixed.
6
Record the time of the first appearance of the dark blue color in seconds.
Part 1 Data Analysis: To Determine m and n From Your Measured Times (Parts 1a and 1b)
7
Calculate [S2O82−] and [I−]
to two significant figures using M1V1 = M2V2
to find M2 for each trial. Note that V2 is the final volume (13 mL).
In Part 1a (trials 1a1 - 1a5), [S2O82−]
varied; in Part 1b (trials 1b1 - 1b5), [I−]
varied.
8
For each run, determine the rate to two significant figures using Rate =
. [S2O32−]
stays the same (use two significant figures); calculate it using | [S2O32−]initial |
| 2t |
M1V1 = M2V2.
Time, t, is the only value that changes trial to trial.
9
To find m, use part 1a data. Take the logarithm of the rate equation.
( 8a )
| log(Rate) | = | m log [S2O82−] | + | log(k[I−]n) |
| y | = | mx | + | b |
10
Plot log(rate) vs. log [S2O82−].
Your log valuse should have two decimal places. Graph your data, draw the best-fit straight line, and determine the slope (which equals m). Round m to the nearest integer.
11
To find n, use part 1b data. Take the logarithm of the rate equation.
( 8b )
| log(Rate) | = | n log [I−] | + | log(k[S2O82−]m) |
| y | = | mx | + | b |
12
Plot log(rate) vs. log [I−].
Your log values should have two decimal places. Graph your data, draw the best-fit straight line, and determine the slope (which equals n). Round n to the nearest integer.
13
To find the rate constant at room temperature for each trial, use a rearrangement of the rate equation.
( 9 )
k =
=
| Rate |
| [S2O82−]m[I−]n |
| [S2O32−]/2t |
| [S2O82−]m[I−]n |
Substitute in your values of m and n, along with the correct time and concentrations for each trial.
Calculate values of k for each of the ten room-temperature runs to two significant figures; then find the average value of k and its standard deviation. Do the values of k differ very much? Should they?
Part 2: Effect of Temperature on the Reaction Rate
Initial Part: Begin this immediately so that the solutions reach the temperature of the water baths.
You already have the data for run #2 of Part 1a (room temperature). Use the concentrations listed in Part 1a, trial #2 to find the rate at 0°C, 35°C, and 45°C.1
Into one test tube (labeled with contents and your name) pipet the reagents given in the table for run #2 of Part 1a.
2
Into another test tube (similarly labeled) pipet 5 mL of 0.20 M KI solution.
3
Stopper them both.
4
Place the pair in an ice water bath made in a 250 or 400 mL beaker.
5
Repeat the process with two test tubes and place them in the 35°C temperature bath.
6
Repeat the process once more and place the tubes in the 45°C temperature bath.
Final Part: Be sure to record the temperature of each bath.
7
Remove a pair of tubes from each bath.
8
Begin to record the time as you add the 5 mL of KI from one test tube into the other.
9
Stopper the tube and gently invert it to mix. Put it back into the water bath.
10
Record the time when you see the onset of the blue color.
The low-temperature run may take 20 minutes or more – keep your eye on it, but do other things while you wait.
11
Repeat for both the other baths.
12
Record room temperature.
Part 2 Data Analysis: Ea and A Determination (temperature dependence of the rate)
1
In addition to the value of k obtained above at room temperature, calculate the value of k to two significant figures for the runs at 0°C, 35°C, and 45°C. (See Equation 9k =
=
| Rate |
| [S2O82−]m[I−]n |
| [S2O32−]/2t |
| [S2O82−]m[I−]n |
2
You now have four data points consisting of k and the corresponding T (in Kelvin). To perform the graphical analysis, take the ln of both sides of the Arrhenius equation and rearrange the following.
( 10 )
| k | = | Ae−Ea/RT | ||||||||||
| ln k | = |
| + | ln A | ||||||||
| y | = | mx | + | b |
3
Plot ln k vs 1/T; ln k should contain two decimal places, 1/T should contain three. You should have four points. The slope of the best-fit line should be straight and equal to –Ea/R; report your slope to three significant figures. The y-intercept should be ln A.
4
Determine the activation energy, Ea, to three significant figures in kJ/mol; R = 8.314 J/K · mol. Calculate the pre-exponential factor, A to two significant figures.
5
Write the complete rate law, substituting your experimental values for m, n, Ea, and A.
Results
Complete your lab summary or write a report (as instructed).Abstract
Results
Tables including concentrations, times, temperatures and rates
Graphs for part 1 and 2
Report the Arrhenius Equation for the reaction (including reaction orders, pre-exponential factor, and activation energy)
Sample Calculations
part 1a: rate, log(rate),
[S2O82−], log [S2O82−];
slope determination
part 1b: rate, log(rate),
[I−] log [I−]
slope determination
part 1: individual room temperature rate constants with average and error
part 2: k, ln k, 1/T, slope of graph, A determination
Discussion/Conclusions
What did you find out and how?
What could be done to improve the accuracy of the experiment?