Significant Figures
Significant Figures In Calculations
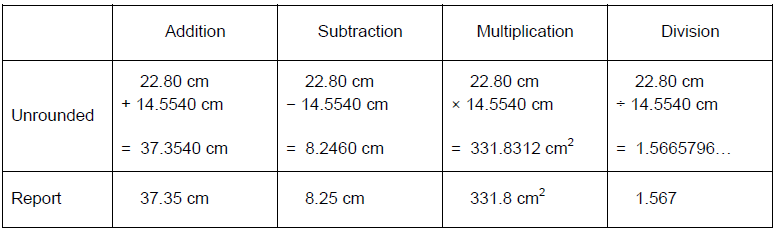
Values cannot become more precise by mathematical manipulation. The following are purely mathematical rules for significant figures in the absence of experimental limitations on precision. Addition and subtraction-
•The final answer is rounded to the least precise decimal place.
-
•The final answer is rounded to the least number of significant figures.
Significant Figures In Experiments
A measured quantity is usually reported as the value and its associated uncertainty. In this class, the following generic symbols are used: x for the value, <x> for the average, and σ for the uncertainty or standard deviation. Please note that uncertainty and standard deviation are technically not the same, but this idea will be covered in more advanced classes. For a single measurement of one quantity, the uncertainty is generally determined by the instrument used.- The pan balance reads to 0.01 g, so an example data would be: 25.10 ± 0.01 g.
- The analytical balance reads to 0.0001 g, so an example would be: 25.1000 ± 0.0001 g.
- A 100-mL graduated cylinder might read to 0.5 mL, so examples are: 25.0 ± 0.5 mL or 31.0 ± 0.5 mL.
( 1a )
<x> =
(x1 + x2 +  + xn) + xn) |
| n |
( 1b )
σ ≡
.
 |
|
Mathematical Manipulations Of Data And Error Propagation
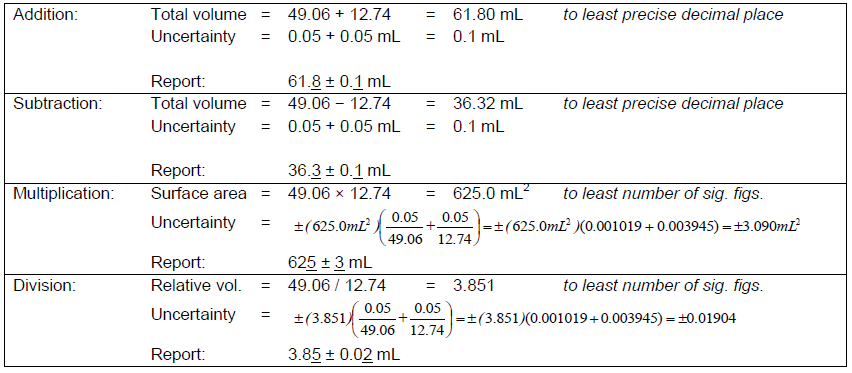
For calculations involving the addition, subtraction, multiplication, and division of two numbers, A ± σa and B ± σa, the following conventions are followed. Addition and subtraction—uncertainties add.( 2 )
Addition: (A ± σa) + (B ± σb) = (A + B) ± (σa + σb)
( 3 )
Subtraction: (A ± σa) − (B ± σb) = (A − B) ± (σa + σb)
( 4 )
Multiplication: (A ± σa)(B ± σb) = (AB) ± (AB)
+
 |
| σa |
| A |
| σb |
| B |
 |
( 5 )
Division:
=
±
+
| (A ± σa) |
| (B ± σb) |
 |
| A |
| B |
 |
 |
| A |
| B |
 |
 |
| σa |
| A |
| σb |
| B |
 |
( 6 )
| σa |
| A |
| σb |
| B |
( 7 )
| σa |
| A |
| σb |
| B |