Experiment 5 - Nitration of Methyl Benzoate
Objective
-
•to demonstrate "Electrophilic Aromatic Substitution"
-
•to provide experience with small-scale synthetic methods
Introduction
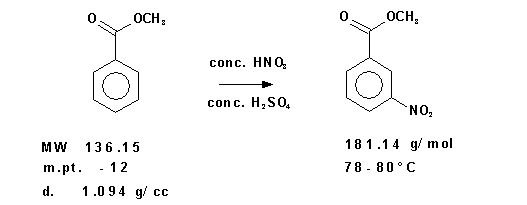
Figure 1
( 1 )
2 H2SO4 + HNO3 → NO2+ + H3O+ + 2 HSO4–Caution:
Concentrated Nitric (HNO3) and Sulfuric Acids (H2SO4) are highly corrosive! Any spills should be promptly flushed with cold water. Discoloration of the skin will result from even a drop of nitric acid.
Concentrated Nitric (HNO3) and Sulfuric Acids (H2SO4) are highly corrosive! Any spills should be promptly flushed with cold water. Discoloration of the skin will result from even a drop of nitric acid.
Pre-Lab
Complete the pre-lab assignment in WebAssign.Procedure
A 1.0 mL sample of conc. Sulfuric Acid is placed in a clean, dry 6" Test Tube and cooled to about 0°C. by swirling in an ice bath. Methyl benzoate (0.7 mL.) is then carefully added. The mixture is shaken to produce one layer. The solution is continuously cooled at 0°C. In a separate 6" Test Tube, prepare a mixture of 0.4 mL of conc. Nitric Acid and 0.4 mL of conc. Sulfuric Acid. Cool this mixture to 0°C. Using a Pasteur Pipet, add the cold mixture of acids drop by drop to the cold solution of the ester in sulfuric acid. Continuously swirl the ester solution in the ice bath as you add the mixture of acids. Keep the temperature of the reaction at about 0°C throughout the reaction! This should take 5-10 min. After the mixed acids have been added, swirl the Test Tube in the ice bath for another 5-10 minutes. Finally, allow the reaction mixture to stand at room temperature, with occasional swirling, for another ten minutes. Fill another 6" Test Tube about two-thirds full with crushed ice. Slowly add a drop or two (Pasteur Pipet) of the reaction mixture onto the ice with swirling. Once crystals of the product begin to form, slowly add the remainder of the reaction mixture, dropwise with swirling. Allow the ice to melt. Collect the solid material by suction filtration on a Hirsch Funnel. Carefully rinse the solid on the filter with 0.5 - 1.0 mL of cool tap water to remove traces of acid. Chill about 0.5 mL of 95% Ethanol to about 0°C. Use this "ice-cold" ethanol to carefully wash the solid on the filter. Draw air through the solid to hasten the drying process. When dry, determine the weight and melting range of the solid. This is the "crude Yield". The solid can be recrystallized from 95% Ethanol. The recrystallized material is your "Purified" Yield. Determine its weight and melting point.Caution:
Ethanol is "FLAMMABLE". Use extreme caution in recrystallizing with this or any other flammable solvent.
Ethanol is "FLAMMABLE". Use extreme caution in recrystallizing with this or any other flammable solvent.
Waste Disposal
The aqueous and ethanol washings from above are combined in a 100 mL beaker. The pH is adjusted to lie between 5.5 and 10.5 by the careful addition of 5% NaOH with continuous stirring. The resulting material, which contains no hazardous materials, may be washed down the drain with a continuous stream of cold water.In-Lab Questions
Download and print the worksheet. You will use this worksheet to record your answers to the In-Lab questions.Questions
Record the following data.- Question 1: Amount of methyl benzoate _____________ mL, _____________ g, ______________ mol
- Question 2: Amount of concentrated nitric acid (16 M) ______________ mL, ______________ mol
- Question 3: Amount of concentrated sulfuric acid (18 M) ______________ mL, ______________ mol
- Question 4: Show your calculations.
- Question 5: Theoretical Yield of methyl 3-nitrobenzoate _________________ mol, __________________ g
- Question 6: Actual Yield of product ___________________ g
- Question 7: Percentage Yield ___________________
- Question 8: Melting Point of product (observed) ___________________
- Question 9: Reported Melting Point of methyl 3-nitrobenzoate ___________________

