Experiment 6 - Aldol Condensation
Objective
To provide experience with Aldol condensation, a useful reaction to prepare conjugated carbonyl systems.Introduction
Hydrogen atoms that are located on a carbon adjacent (alpha) to a carbonyl group are acidic and can be removed by base. The acidity is due to the fact that the carbanion produced is stabilized by resonance with the carbonyl group (1 and 2). The carbanion is a nucleophile and is capable of adding to electrophilic centers such as the carbon of the carbonyl group in aldehydes and ketones. The result of such a reaction is the formation of a beta-hydroxy carbonyl compound (3). In cases where there are aromatic substituents, these initial products undergo dehydration to yield the conjugated system (4).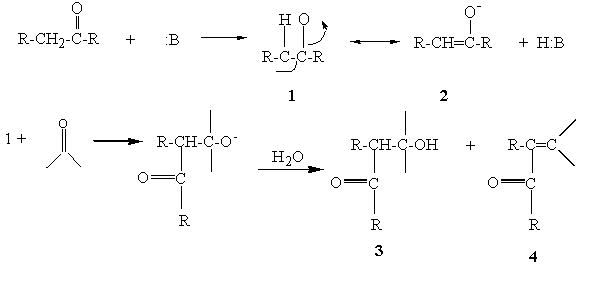
Figure 1
Pre-Lab
Complete the pre-lab assignment in WebAssign.Preparation of Dibenzalacetone
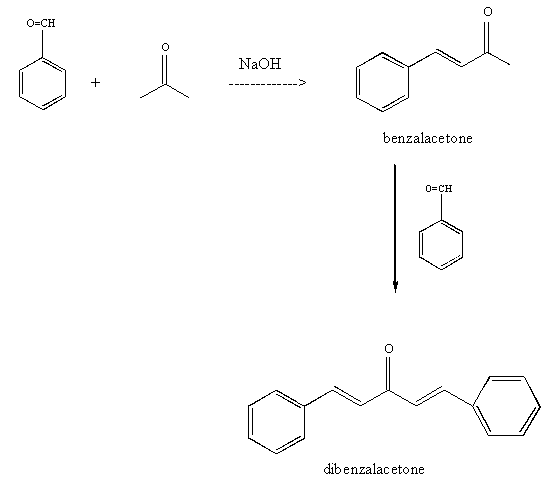
Figure 2
Procedure
In a small Erlenmeyer flask, mix 2 mL of the sodium hydroxide solution with 2 mL of 95% ethanol.Caution:
The NaOH solution is about 20%; avoid contact. Wash hands after use!
The NaOH solution is about 20%; avoid contact. Wash hands after use!
In-Lab Questions
Download and print the following worksheet. You will use this worksheet to record your answers to the In-Lab questions.Questions
Record the following data.- Question 1: Amount of benzaldehyde ______________ mL, ______________ g, _______________ mol
- Question 2: Amount of acetone _______________ mL, _______________ g, ________________ mol
- Question 3: Theoretical Yield of Product __________________ mol, __________________ g
- Question 4: Actual Yield of Product ____________________
- Question 5: Percentage Yield ____________________
- Question 6: Melting Point of Product __________________ (observed), __________________ (reported)
- Question 7: Record your calculations.
- Question 8: Record the mechanism of reaction.

