Lab Investigation 8 - What shapes do molecules form?
Lewis dot structures help us predict covalent bonding patterns as well as locate non-bonding pairs of electrons on a molecule. Given a correct Lewis dot structure we can predict the shape of a molecule using Valence Shell Electron Pair Repulsion theory. Predicting the shape of a molecule is important for understanding how that molecule interacts with other molecules such as enzymes or antibiotics. Finally, knowing the bonding and the shape of a molecule enables us to predict polarity. Polarity is vital to understanding how molecules interact with each other. In this laboratory exercise you will have the opportunity to use models to build molecules of all shapes and bonding. You will predict the shape of molecules using your model.Question 1
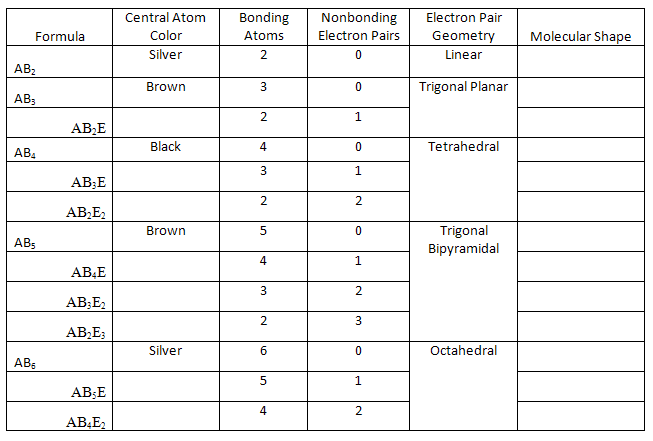
First, we will become familiar with the model kit by constructing the 5 basic shapes for compounds in which all electrons are involved in bonds. Construct each of the models below. A is the central atom in each case.Question 2
The electron pairs around a central atom will always have one of the five basic shapes you made models of in question 1. If one or more of the electron pairs is non-bonding, we describe the shape of the molecule based on the relative position of the bonding groups. This is called the molecular shape as opposed to the electron pair geometry. We will systematically look at the effect of a lone pair of electrons on each type of geometry. The letter E indicates a non-bonding pair of electrons. Construct a model for each formula; you can just use an empty bond for the lone pair. Fill in the name for the molecular shape.Question 3
Use the set of molecular models to construct each of the following molecules. Identify each of the items listed.-
aBeCl2
-
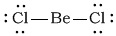
-
Bonding Atoms Non-bonding Electron Pairs Electron Pair Geometry Molecular Shape Polarity
-
bCO2
-
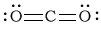
-
Bonding Atoms Non-bonding Electron Pairs Electron Pair Geometry Molecular Shape Polarity
-
cHCN
-
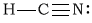
-
Bonding Atoms Non-bonding Electron Pairs Electron Pair Geometry Molecular Shape Polarity
-
dXeCl2
-
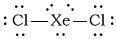
-
Bonding Atoms Non-bonding Electron Pairs Electron Pair Geometry Molecular Shape Polarity
Question 4
Use the set of molecular models to construct each of the following molecules. Identify each of the items listed.-
aSO2
-
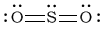
-
Bonding Atoms Non-bonding Electron Pairs Electron Pair Geometry Molecular Shape Polarity -
bH2S
-
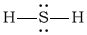
-
Bonding Atoms Non-bonding Electron Pairs Electron Pair Geometry Molecular Shape Polarity
Question 5
Use the set of molecular models to construct each of the following molecules. Identify each of the items listed.-
aSiF4
-
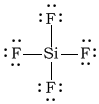
-
Bonding Atoms Non-bonding Electron Pairs Electron Pair Geometry Molecular Shape Polarity -
bXeF4
-
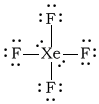
-
Bonding Atoms Non-bonding Electron Pairs Electron Pair Geometry Molecular Shape Polarity -
cSF4
-
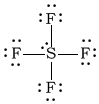
-
Bonding Atoms Non-bonding Electron Pairs Electron Pair Geometry Molecular Shape Polarity
Question 6
Use the set of molecular models to construct each of the following molecules. Identify each of the items listed.-
aNO3–1
-
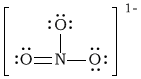
-
Bonding Atoms Non-bonding Electron Pairs Electron Pair Geometry Molecular Shape Polarity -
bPF3
-
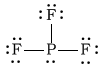
-
Bonding Atoms Non-bonding Electron Pairs Electron Pair Geometry Molecular Shape Polarity -
cCH2O
-
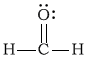
-
Bonding Atoms Non-bonding Electron Pairs Electron Pair Geometry Molecular Shape Polarity