Lab 11 - Thermodynamics of Salt Dissolution
Organization
-
•Mode: Part A groups of 3 or 4; Part B individual work; Part C back to groups
-
•Grading: lab notes, lab performance, and post-lab report
-
•Safety: goggles, closed-toe shoes, long pants/skirts, and sleeves
Goal:
To use calorimetry to calculate enthalpies of dissolution for several salts and to rationalize the trends using intermolecular forces between the salts and water.
To use calorimetry to calculate enthalpies of dissolution for several salts and to rationalize the trends using intermolecular forces between the salts and water.
| CHEM 115 Expt. 11 | Chemical Classification | Possibility Of: | NFPA Codes | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Thermodyanmics of Salt
Dissolution |
Poison A
|
Flammable Gas
|
Flammable Liquid
|
Combustable Liquid
|
Reacts with Water
|
Oxidizer
|
Organic Peroxide
|
Poison B
|
Corrosive Acid
|
Corrosive Base
|
Irritating or Harmful
|
Misc. Hazard
|
No Hazard
|
Fire
|
Sudden Release of Pressure
|
Reactive
|
Immediate (Acute) Health Hazard
|
Delayed (Chronic) Health Hazard
|
Fire
|
Health
|
Reactivity
|
Special Precautions
|
| Ammonium Nitrate | X | 0 | 1 | 3 | ||||||||||||||||||
| Calcium Chloride, Anhydrous | X | 0 | 1 | 1 | ||||||||||||||||||
| Magnesium Sulfate | 0 | 1 | 0 | |||||||||||||||||||
| Sodium Acetate | X | 0 | 1 | 0 | ||||||||||||||||||
| Sodium Carbonate | X | 0 | 1 | 1 | ||||||||||||||||||
| Sodium Chloride | X | 0 | 1 | 0 | ||||||||||||||||||
| Sodium Nitrate | X | 0 | 1 | 3 | ||||||||||||||||||
| Magnesium Carbonate | X | 0 | 1 | 1 | ||||||||||||||||||
I: Background
In a spontaneous reaction, reactants are converted to products without any outside influence. To roll a rock from the top of a hill to the bottom of a hill, one needs only to tip the rock over the edge, and the rock converts from reactant (rock at the top of the hill) to product (rock at the bottom of the hill) with no additional help. This is an example of a spontaneous process. Going the other way, however, from the rock at the bottom of a hill to the rock at the top of a hill, is not a spontaneous process and requires continuous outside influence, in the form of someone pushing the rock up the hill. Generally, the kind of outside influence required to drive a non-spontaneous process is the continuous input of energy. The term "spontaneous" applied to chemical reactions is specific to the formation of products from reactants. Another term used for the spontaneous conversion of reactants to products is "product-favored". Thermodynamics is the field of study of heat (thermo), and work (dynamics), i.e. the flow of energy, that occurs during chemical or physical change. The change in energy that accompanies a chemical or physical change determines whether or not that change is spontaneous or product-favored. Sometimes energy is released during a chemical or physical change (burning gasoline or wood gives off heat), and sometimes energy is absorbed during a chemical or physical change (solid water absorbs heat to melt to liquid). When energy is released during a chemical or physical change, energy can be thought of as a product of the process. When energy is absorbed during a chemical or physical change, energy can be thought of as a reactant for the process. One component of the thermodynamic change associated with a chemical or physical change (such as a phase change) is the enthalpy change, ΔH and the other component is the entropy change, ΔS. Each of these terms contributes to the thermodynamic fate of a chemical or physical change, but it is the combination of these terms that determines if a change is product-favored or not. Many product-favored processes are ones in which the products are lower in energy than the reactants; like a rock on a hill, the spontaneous direction of change is to a lower energy state. We won't be discussing entropy in CHEM 115, but will focus solely on enthalpy. Thermodynamics uses common English words and terms, but with the field's own very specific meanings for those words and terms. We have to know the thermodynamic meaning of the terms used to begin a discussion of the enthalpy changes associated with a chemical process.Systems and Surroundings
The system is defined as the chemicals involved in a chemical reaction or a phase change. The surroundings are defined as everything around the system, including the solvent, beakers, thermometers, the room, and the experimenter. All analyses of thermodynamic parameters are done from the perspective of the system, the chemical reaction taking place, but all measurements are done from the surroundings. For example, an increase in temperature may occur during a chemical reaction. This increase is measured in the surroundings using a thermometer, but the interpretation of this temperature change is that the system is giving off heat to the surroundings, causing the temperature in the surroundings to increase. Using the symbol q to denote the flow of heat, we can write this relationship as qsystem = –qsurroundings.ΔH, Enthalpy Change and Thermochemistry
Thermochemistry is the study of energy change associated with chemical reactions. Many chemical reactions are accompanied by the exchange of energy in the form of heat between the system and the surroundings. When reactions are carried out at constant pressure (for example, in open containers in a chemistry lab), the heat change is proportional to the enthalpy change, ΔH. By convention, if heat is released from a chemical system to the surroundings, that reaction is classified as exothermic, and the sign of the enthalpy change is negative (ΔH < 0, exothermic, heat released from system). If heat is absorbed by the system from the surroundings, that reaction is classified as endothermic, and the sign of the enthalpy change is positive (ΔH > 0, endothermic, heat absorbed by system). Heat changes are easily measured in the surroundings using a thermometer. Think for a moment how these enthalpy changes would be measured in the surroundings. If a reaction is exothermic, would the surroundings experience an increase or a decrease in heat? Would this be measured as an increase or decrease in temperature? Product-favored processes are those in which energy is released as the reactants form products. Exothermic reactions (ΔH < 0) tend to favor the formation of products, but the sign of ΔH by itself does not determine whether or not product will be formed spontaneously.Using the Dissolution of Ionic Compounds in Water to Explore Thermodynamics
Some ionic compounds dissolve readily in water, while others are insoluble. Some ionic compounds give off heat when dissolving in water and others absorb heat. Whether the dissolution process of a given ionic compound gives off or absorbs heat depends on the strength of the intermolecular forces holding the solid together, as well as those between the ions and the water once dissolved. In this exercise, you will examine the dissolution of various salts in water.Intermolecular Forces Contribute to the Enthalpy of a Solution
During the dissolution of a solute in a solvent, there are attractive forces between the solute particles that must be disrupted, and attractive forces between the solvent molecules that must be disrupted (Figure 1). For a solution to form, intermolecular forces must form between the solute and the solvent (Figure 1). A solution will form only if the attractive forces between the solute particles and the solvent molecules are similar in strength and kind as the solute-solute intermolecular forces and the solvent-solvent intermolecular forces. The solute particles will only associate with solvent particles if they "like" the solvent particles as much as or more than they "like" the other solute particles: Like dissolves like.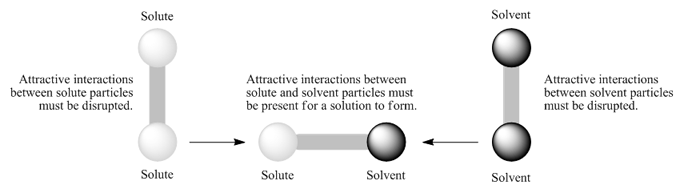
Figure 1
( 1 )
| ΔHrxn | = | Σ(bond energy of solute-solute + solvent-solvent attractive forces) |
| – | Σ(bond energy of solute-solvent attractive forces) |
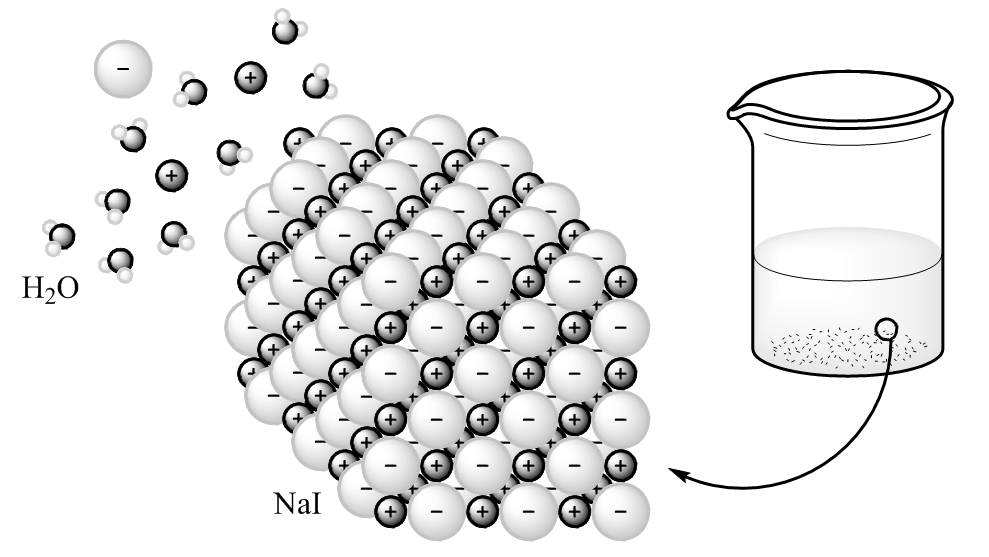
Figure 2
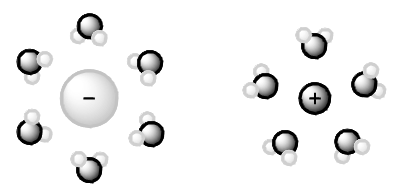
Figure 3
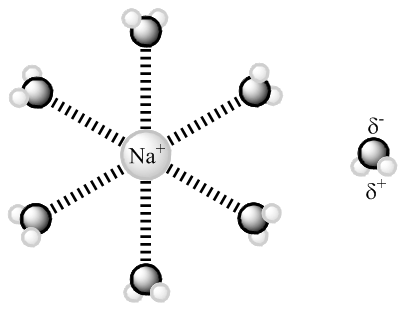
Figure 4
II: Exercises
Part A: Predicting the Solubility for the Dissolution of Salts
Reproduce Table 1 in your notebook.|
Ionic
Compound |
Formula |
Solubility
Prediction |
Experimental Result:
Soluble, Partially Soluble or Insoluble |
Product or
Reactant Favored |
|---|---|---|---|---|
| ammonium nitrate | ||||
| calcium chloride | ||||
| magnesium sulfate | ||||
| sodium acetate | ||||
| sodium carbonate | ||||
| sodium chloride | ||||
| sodium nitrate | ||||
| magnesium carbonate |
1
Provide the formula for each ionic compound in the table.
2
Write the balanced reaction for the dissolution of each salt in water, including physical states. The convention is to leave water out of the equation, and simply write the reactant as the solid ionic compound and the product as ions in aqueous solution. Below is an example of a reaction for the dissolution of an ionic compound in water.
When a soluble ionic compound is placed in water, the solid is converted to the product of the dissolution reaction—the solid vanishes, converting to dissolved ions in solution.
3
Use the solubility rules to determine if the salt is soluble or insoluble, and enter that information in the table.
4
For each ionic compound, decide if the dissolution reaction is product-favored or not, and enter that information in the table.
In the language of thermodynamics, we would say that the dissolution reaction is product-favored if the product is (predicted to be) soluble.
5
Test your prediction. Dividing the work among 4 people, fill one test tube about half-way with distilled water for each salt, and put a small spatula full of salt into each test tube. Shake to stir, or stir with the stirring rod.
6
Discuss the following with your group for each salt.
-
a.Did a dissolution reaction occur?
-
b.Was the reaction product-favored?
-
c.What was your evidence?
Part B: Observations
Temperature Changes Upon Dissolution of Salts Each student performs Part B individually. The salts to be investigated are ammonium nitrate, calcium chloride, magnesium carbonate, and a fourth salt of your choice—either sodium chloride, sodium nitrate, magnesium sulfate, sodium acetate, or sodium carbonate. Reproduce Table 2 below to record your observations for this part of the exercise. As always, all observations, interpretations of those observations, data, and answers to questions in the exercise must be recorded in your laboratory notebook.| Ionic Compound |
Mass
(g) |
Ti
(°C) |
Tf
(°C) |
ΔT = Tf – Ti
(°C) |
qreaction
(J) |
ΔHreaction
(J/mol) |
|---|---|---|---|---|---|---|
| ammonium nitrate | ||||||
| calcium chloride | ||||||
| magnesium carbonate | ||||||
| salt of your choice |
1
Prepare a coffee-cup calorimeter by placing one Styrofoam coffee cup inside another.
2
Use a graduated cylinder to measure 50.0 mL of cold tap water and pour it into the coffee cup. Record the temperature of the water.
3
Weigh out ~5 grams of ammonium nitrate. Record the weight of the salt. Add the salt to the water and stir to dissolve—use a stirring rod, not the thermometer to stir. Observe the temperature changes over time that occur while the salt dissolves. Record the highest or lowest temperature observed as the final temperature.
4
Perform this experiment for the other three salts—however, use only ~2 grams of magnesium carbonate, but ~5 grams of the other salts.
5
For your lab report, use the following information to calculate the heat transferred during the dissolution of your salts and the molar enthalpy change of the dissolution. Include appropriate signs.
Part C: Analysis of the Dissolution Process and the Change in Enthalpy
Get together in groups of 3 or 4 students. Compare results from Part B, and repeat any salt dissolution if there are discrepancies. For each salt in turn, consider the reaction that occurred when the salt was added to water.-
a.For each reaction, identify the solute and the solvent.
-
b.What types of intermolecular forces occur between the solute particles?
-
c.What types of intermolecular forces occur between the solvent particles?
-
d.What types of intermolecular forces occur between solute and solvent particles?
-
e.Review the classification of each dissolution as endothermic or exothermic and the sign of ΔH.
