Exploration of Reaction Kinetics
Organization
-
•Mode: laboratory work, work in pairs
-
•Grading: lab notes, lab performance, and post-lab report
-
•Safety: goggles, closed-toe shoes, long pants/skirt, and sleeves; latex or nitrile gloves recommended
Goal:
To identify the factors that can be used to change the rate of a chemical reaction. To predict, when possible, the factors based on the stoichiometry and mechanism of the reaction.
To identify the factors that can be used to change the rate of a chemical reaction. To predict, when possible, the factors based on the stoichiometry and mechanism of the reaction.
| CHEM 115 Expt. 12 | Chemical Classification | Possibility Of: | NFPA Codes | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reaction Kinetics |
Poison A
|
Flammable Gas
|
Flammable Liquid
|
Combustable Liquid
|
Reacts with Water
|
Oxidizer
|
Organic Peroxide
|
Poison B
|
Corrosive Acid
|
Corrosive Base
|
Irritating or Harmful
|
Misc. Hazard
|
No Hazard
|
Fire
|
Sudden Release of Pressure
|
Reactive
|
Immediate (Acute) Health Hazard
|
Delayed (Chronic) Health Hazard
|
Fire
|
Health
|
Reactivity
|
Special Precautions
|
| Oxalic Acid, 0.20 M | X | X | X | 0 | 2 | 1 | ||||||||||||||||
| Potassium Permanganate, 0.0015 M | X | 0 | 2 | 2 | ||||||||||||||||||
| Sulfuric Acid, 2.0 M | X | X | X | 0 | 2 | 1 | COR | |||||||||||||||
I: Background
In order for a reaction to take place, the molecules, atoms, or ions involved in that reaction must collide in the proper orientation and with sufficient energy. Only a small fraction of the collisions that take place result in a reaction occurring. The rate of a reaction is therefore directly influenced by the number of collisions that occur, and the energy of those collisions. By manipulating either the collision frequency or the collision energy, the rate of a chemical reaction can be manipulated. In the previous exercise, you manipulated the rate of a reaction by changing the concentration of reactant. Would this change have an effect on collision energy or collision frequency? What very simple thing can be done in a laboratory to manipulate the energy of chemicals in a reaction flask?
During the course of this exercise, you will be exploring the following questions.
-
1.What factors can be exploited to change the rate of a chemical reaction?
-
2.Can these factors be predicted from the stoichiometry of the chemical equation?
-
3.Why do these factors change the rate of a chemical reaction?
-
•the concentrations of the reactants and products
-
•the concentrations of spectator ions (ionic strength of the solution)
-
•the concentration or surface area of a catalyst
-
•the pressure of the reaction vessel
-
•the temperature at which the reaction is performed
| 2 MnO4–(aq) | + |
5 C2O4H2(aq) | |
6 H3O+(aq) | |
| permanganate ion | oxalic acid | acid, H2SO4 |
Reaction Mechanisms
As an analogy to the concept of a mechanism in chemistry, imagine buying a bookcase that requires assembly (Figure 1).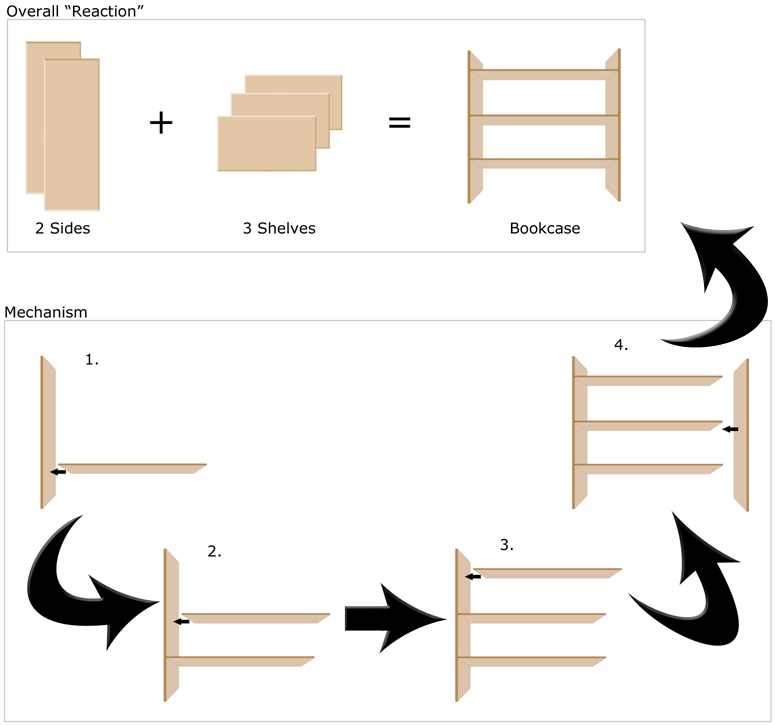
Figure 1: Analogy of Building a Bookcase With Chemical Mechanisms
Activation Energy
In every chemical reaction, the reactants must overcome an energy barrier in order to react. This energy barrier is called the activation energy and is abbreviated Ea. Shown in Figure 2 are two reaction progress diagrams. The energy of the chemical species as the reaction progresses is traced on the y-axis. The reactants must gain energy to reach the transition state, after which it is possible for the reactants to be converted to products. Both the activation energy and the net change in energy can be deduced from Figure 2.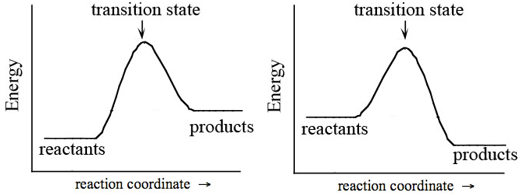
Figure 2: Reaction Progress Diagrams for Single-Step Endothermic (Left) and Exothermic (Right) Reactions
Effect of Temperature on Kinetics
While an increase in collision frequency also results in higher temperature, it is not the number of collisions that determines the rate of the reaction—it is the energy of the collision that determines the rate of the reaction. You can hit a pool ball softly over and over and over again, requiring many collisions before you sink it, or you can hit it hard once and sink it. It is the energy of the collision that matters most, and temperature is the way to control the energy of the collision. As the temperature increases, the number of collisions occurring with energy equal to Ea increases exponentially, and that is why the rate of the reaction increases with increasing temperature.Mechanisms and Rate-Limiting Steps
What does it mean if the rate of a reaction does not depend on the concentration of one of the reactants present in the overall equation?
The slowest step in the mechanism is called the rate-limiting step, and this step determines the rate of the overall reaction.
II: Exercises
Part A: Getting Ready to Perform the Experiments
1
Prepare four clean, dry, labeled 50 mL beakers, one for each of the following four solutions. Obtain ~30 mL of each solution.
-
•0.0015 M KMnO4, potassium permanganate
-
•0.20 M C2O4H2, oxalic acid
-
•2.0 M H2SO4, sulfuric acid (used for H3O+(aq) in the reaction)
-
•distilled water
2
Label four pipettes, one for each of the four solutions identified in Step 1. You will use these pipettes to prepare various mixtures of the four solutions.
3
Clean, rinse in distilled water, and dry twelve 3-inch or 4-inch test tubes. Place the test tubes in a rack.
Part B: Exploring the Redox Reaction
1
Label three clean test tubes. Support the test tubes in a test tube rack, and place a piece of white paper underneath and behind the test tubes.
2
Try to be consistent in the size of drops delivered to each test tube. Make sure the drop isn't just a bubble of solution.
3
Make up the three solutions as described below, one in each test tube. After adding all of the reagents, mix each solution by flicking the bottom of the test tube with your index finger, while firmly holding the top of the test tube. Observe the test tubes for about ten minutes. Record your observations in your laboratory notebook.
-
•To test tube B-1, add 15 drops of KMnO4 and 15 drops of H3O+/H2SO4.
-
•To test tube B-2, add 15 drops of KMnO4 and 5 drops of C2O4H2.
-
•To test tube B-3, add 15 drops of KMnO4, 15 drops of H3O+/H2SO4, and 5 drops of C2O4H2.
4
Discuss your observations, and think about the reaction using the following questions.
-
a.Why didn't a reaction occur in each test tube?
-
b.For a test tube in which a reaction occurred, how did you judge that the reaction was taking place?
-
c.Did the reaction take place instantaneously, or did it take time to complete?
-
d.What was the evidence that the reaction took time to complete?
Exploring Ways to Increase the Rate of a Chemical Reaction
General Experimental Notes The rest of the lab involves exploring different experimental parameters on the rate of the reaction explored in Part B. For each of the following experiments, you will use three 4-inch test tubes that have been cleaned, distilled-water rinsed, dried, and labeled. Support the test tubes in a test tube rack, and place a piece of white paper underneath and behind the test tubes. Unlike Part B, you must carefully record the time it takes for the entire permanganate ion sample to be reduced by the oxalic acid. The recommended procedure is as follows.1
Create a table in your laboratory notebook to record the contents of each test tube and the time of each reaction.
2
Set up the test tubes in the test tube rack.
3
Add the indicated quantities of all of the stock solutions, except for the oxalic acid solution, to the test tubes.
4
One member of the team has the job of starting the reaction by adding the oxalic acid solution to the test tubes and saying when the reaction is over.
5
The other member of the team has the job of recording the time it takes for the reaction to finish.
6
Be consistent from experiment to experiment on the color that signifies the end of the reaction.
7
Empty the contents of the test tubes into a beaker at your bench for later disposal in the hazardous waste container.
Part C: Exploring the Effect of the Concentration of H3O+(aq)
1
Prepare three test tubes with the drops of KMnO4, H2O, and H3O+ (delivered as H2SO4), as indicated below.
-
•To test tube C-1, add 15 drops of KMnO4, 25 drops of H2O, 5 drops of H3O+/H2SO4, and 5 drops of C2O4H2.
-
•To test tube C-2, add 15 drops of KMnO4, 15 drops of H2O, 15 drops of H3O+/H2SO4, and 5 drops of C2O4H2.
-
•To test tube C-3, add 15 drops of KMnO4, 5 drops of H2O, 25 drops of H3O+/H2SO4, and 5 drops of C2O4H2.
If you are using a stop watch or the second hand of a watch to record the time it takes for the reaction to end, you will have to observe each reaction one at a time. If you want to do all three reactions in a more simultaneous fashion, record the actual time when reactions start and stop. In this way, you can have all reactions running at the same time, and you can use the start and stop times to calculate the time of the reaction.
2
Start the reaction in the first tube by adding the indicated number of drops of C2O4H2 and flicking the bottom of the test tube, or shaking it to mix the contents. Begin recording the time for this reaction. Repeat for the next two tubes.
3
When the reaction in each test tube is over, record the end time of the reaction. Record the total time it took for the reaction in each test tube to end.
4
Data Analysis and Discussion:
-
a.Is H3O+ a reactant or a product in the reaction? What effect did increasing the amount of H3O+ have on the rate of the reaction?
-
b.Which test tube had the slowest reaction rate, and which had the fastest reaction rate? How does the reaction time relate to rate of reaction—do long reaction times reflect slow reaction rates or fast reaction rates?
While we are not explicitly measuring the rates of these reactions, you can use the time of the reaction to determine the relative rate of reaction.
-
c.By what factor did the concentration of H3O+ change from C–1 to C–2. By what factor did the rate of the reaction change from C–1 to C–2?
-
d.If the rate of a reaction changes by a substantial amount (factor of 2 or more) when the concentration of a reactant changes, then the rate of the reaction is considered to be dependent on the concentration of that reactant. Would you say that the rate of the reaction depends on the concentration of H3O+ in solution?
-
e.What are the molecules and ions doing in solution—are they static or moving around?
-
f.Can ions or molecules that are isolated from one another react? What must happen for them to react?
-
g.Why should the permanganate disappear faster if the concentration of H3O+(aq) is higher?
-
h.Devise a general, qualitative statement about the effect that an increase in the concentration of a reactant has on the rate of a reaction, and an explanation of that effect.
Part D: Exploring the Effect of Temperature
1
Form a partnership with two other pairs of students. One pair is to do the experiment at room temperature, one pair at a temperature about 10°C above room temperature, and one pair at a temperature about 10°C below room temperature. Each pair will do two trials at the temperature assigned.
2
Prepare a water bath using a half-filled 250 mL beaker. Begin adjusting the temperature of the water bath by either adding ice or heating the water on a hot plate. Add the indicated quantities of the KMnO4, H2O, and H2SO4 solutions to test tubes D–1 and D–2, and place the test tubes in the water bath.
3
Transfer ~1 mL of the C2O4H2 solution into a clean, dry test tube, and place that test tube into the water bath as well. You will add this solution to test tubes D–1 and D–2 to start the reactions.
4
Let these solutions reach thermal equilibrium by sitting in the beakers for ~10 minutes (more time is okay). Monitor the temperature, and heat or cool to keep the temperature within a few degrees of the target temperature.
5
After the solutions have reached thermal equilibrium, perform the reaction. Add the indicated quantity of the C2O4H2 solution to test tube D–1, mix the solution, and quickly put the test tube back into the water bath. Repeat the experiment with the solution in test tube D–2. Record the reaction time and the temperature of the water bath in which the test tubes are sitting.
6
Exchange data with the other groups in your partnership. Include reaction time and temperature, along with the names of the people in the other groups.
7
Data Analysis and Discussion:
-
a.What effect does the temperature of the solutions have on the rate of the reaction?
-
b.How does heating the reaction mixture affect the energy of the species in solution? What effect would cooling the reaction mixture have on the energy of the species in solution?
-
c.Write a general, qualitative statement about how the rate of a reaction depends on the temperature at which the reaction is carried out.
Part E: Exploring the Effect of Copper Metal
1
Prepare three test tubes with the drops of KMnO4, H2O, and H3O+ (delivered as H2SO4), as indicated below. Place the strips of copper in the test tubes. While the size of the copper strips does not matter, in the third test tube, you want to have twice as much copper as in the second test tube. For the test tube with two strips of copper, ensure that they do not touch each other, in order to maximize the surface area in contact with the solution.
-
•To test tube E-1, add 15 drops of KMnO4, 15 drops of H2O, 15 drops of H3O+/H2SO4, and 5 drops of C2O4H2.
-
•To test tube E-2, add 15 drops of KMnO4, 15 drops of H2O, 15 drops of H3O+/H2SO4, 1 strip of copper, and 5 drops of C2O4H2.
-
•To test tube E-3, add 15 drops of KMnO4, 15 drops of H2O, 15 drops of H3O+/H2SO4, 2 strips of copper, and 5 drops of C2O4H2.
2
Start the reaction in the first tube by adding the indicated number of drops of C2O4H2 and flicking the bottom of the test tube, or shaking it to mix the contents. Begin recording the time for this reaction. Repeat for the next two tubes.
3
When the reaction in each test tube is over, record the end time of the reaction. Record the total time it took for the reaction in each test tube to end.
4
Data Analysis and Discussion:
-
a.What effect does the presence and amount of copper have on the rate of the reaction?
-
b.Does it appear that the copper reacted during the course of the reaction?
-
c.Could you have predicted the effect of copper on the rate of this reaction, based on the equation of the overall reaction taking place? Explain your answer.
Part F: Rates of Reaction and Activation Energy
In this exercise, you observed the effect of the concentration of H3O+, temperature, and Cu(s) on the rate of reaction. This was a macroscopic observation based on a change in color that occurred in the test tube. This macroscopic observation tells you what happened to the rate of a reaction as the function of changing amounts or reactants, temperature, or adding Cu, but it doesn't tell you how these changes influence the rate of the reaction. To understand how these changes affect rate, you must think at a molecular level, in terms of collisions between the molecules in the test tubes. In the background for this exercise, you were told that for a reaction to take place, the molecules or ions involved in the reaction must collide in the proper orientation and with sufficient energy. The change in rate that you measured was a macroscopic observation. The relationship between the frequency of collisions and concentration of reactants was explored in Part C; and in questions e–h, you thought about how a change in H3O+ affected rate at a molecular level. In this section, you will explore the relationship between the rate of a reaction and the energy of a collision, and the effect a catalyst has on the rate of a reaction. Consider the following figure. The darker curve represents the uncatalyzed reaction; the lighter line represents the catalyzed reaction.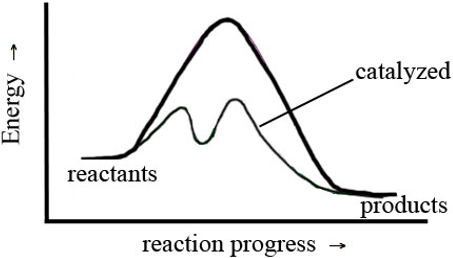
Figure 3: The Influence of Catalysts on Reaction Mechanisms and Activation Energy
-
1.In Figure 2, which reaction requires more energy to reach the transition state? Recreate the diagrams in your notebook. On each diagram, draw a line indicating where you measured this energy, in order to make the comparison. This energy is called the activation energy, Ea, and it represents the energy barrier between reactants and products.
-
2.Which reaction in Figure 2 do you think would be faster—one with a larger energy barrier or one with a smaller energy barrier? Explain why you think so. Given this, which reaction profile corresponds to a faster reaction, and which corresponds to a slower reaction?
-
3.For a reaction to take place, the species involved must collide with sufficient energy. What is the minimum amount of energy needed for a collision to result in a reaction?
-
4.Devise a general statement about the relationship between Ea and the rate of a reaction.
-
5.Describe the effect of temperature on the energy of collisions, and explain how this effect changes the rate of a reaction.
-
6.Describe the differences in appearance between the catalyzed and uncatalyzed pathways in Figure 3, including relative energies and number of energy barriers.
-
7.Which reaction will be faster—the catalyzed pathway or the uncatalyzed pathway? Why?
-
8.Of the variables studied during this experiment, H3O+(aq), C2H4O2(aq), Cu(s), and temperature, which was the catalyst? Why do you think so?
-
9.Describe the effect of a catalyst on the rate of a reaction, and explain how a catalyst changes the rate of a reaction.
