Shifting Equilibrium: Le Châtelier's Principle
Organization
-
•Mode: laboratory work, work in pairs or groups of three
-
•Grading: lab notes, lab performance, and post-lab report
-
•Safety: goggles, closed-toe shoes, long pants/skirts, and sleeves; latex or nitrile gloves are recommended; use care with acids and bases
Goal:
To determine the effect that adding or removing reactant or product or changing temperature has on a system in equilibrium. To provide a kinetic explanation for the effect that changes in reactant or product concentration or temperature has on equilibrium.
To determine the effect that adding or removing reactant or product or changing temperature has on a system in equilibrium. To provide a kinetic explanation for the effect that changes in reactant or product concentration or temperature has on equilibrium.
| CHEM 115 Expt. 13 | Chemical Classification | Possibility Of: | NFPA Codes | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Shifting Equilibrium:
Le Châtelier's Principle |
Poison A
|
Flammable Gas
|
Flammable Liquid
|
Combustable Liquid
|
Reacts with Water
|
Oxidizer
|
Organic Peroxide
|
Poison B
|
Corrosive Acid
|
Corrosive Base
|
Irritating or Harmful
|
Misc. Hazard
|
No Hazard
|
Fire
|
Sudden Release of Pressure
|
Reactive
|
Immediate (Acute) Health Hazard
|
Delayed (Chronic) Health Hazard
|
Fire
|
Health
|
Reactivity
|
Special Precautions
|
| Hydrochloric Acid, 12 M | X | X | X | 0 | 3 | 2 | ||||||||||||||||
| Cobalt(II) Chloride, 0.1 M | X | X | 0 | 3 | 0 | |||||||||||||||||
| Silver Nitrate, 0.1 M | X | X | 0 | 2 | 0 | |||||||||||||||||
I: Background
Transition metal complexes are often highly colored. The color is usually the result of electronic transitions from one d orbital to another d orbital; these are called d-d transitions. In this exercise, you will see evidence that a change in the coordination environment of Co2+ results in a change in color, and you'll use this color change to explore Le Châtelier's Principle. You start with an aqueous solution of cobalt(II) chloride. The cobalt ion is present as a hexaaquocobalt(II) complex ion, [Co(H2O)6]2+, in this solution (Figure 1).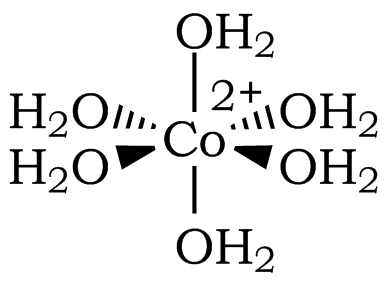
Figure 1: The Octahedral Structure of [Co(H2O)6]2+ (Coordination Number of 6)
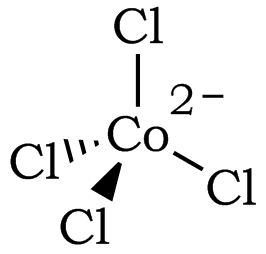
Figure 2: The Tetrahedral Structure of [CoCl4]2– (Coordination Number of 4)
The effect that the addition or removal of a reactant, product, or heat to a system at equilibrium has on the equilibrium of the system is known as Le Châtelier's Principle. Formally, this principle states that if stress is applied to a system in equilibrium, the system will react to relieve that stress. To understand why Le Châtelier's Principle operates, it's helpful to think about what happens to the relative rates of the forward and reverse reactions upon the application of a stress to a system at equilibrium.
II: Exercises
Getting Started
Read through the entire procedure and get enough of the cobalt(II) chloride and concentrated HCl solutions for the entire exercise. Fill two 250 mL beakers half-full of water. Put one on a hot plate and start warming it. Put ice in the other.
All work with concentrated HCl must be carried out in the hood.
Part A: Exploration of the Reaction
1
Place 5 mL of the 0.1 M cobalt(II) chloride solution in a 50 mL beaker. Slowly and carefully add 10-15 mL concentrated hydrochloric acid in ~1 mL aliquots. Record your observations of the initial solution and of how that solution changes as each aliquot of HCl is added. (carry out reaction in hood)
2
Keep the final solution for use in Part D.
Part B: Make the Stock Solution for the Exploration
1
Place 25 mL of the 0.1 M cobalt(II) chloride solution in a 125 mL erlenmeyer flask. Place 25 mL of concentrated HCl in a graduated cylinder. Slowly and carefully add hydrochloric acid to the cobalt(II) chloride solution until the solution turns purple. Record the amount of HCl added. This solution will be used for Parts C, D, and E. Record the color of this solution. (carry out reaction in hood)
Part C: Effect of Silver Nitrate on the Equilibrium
1
Put about 15 mL of the purple solution from Part B into a 50 mL beaker. Place about 5 mL of silver nitrate solution into a clean test tube or small beaker. Add the silver nitrate solution dropwise to the cobalt solution. Continue adding silver nitrate until a change is seen. Record your observations.
Part D: Effect of Water on the Equilibrium
1
Place ~15 mL of the solution from Part B into a 50 mL beaker. Record the color of the solution, and then add an equal volume of water. Describe what happens.
2
Record the color of the solution kept from Part A, and then add an equal volume of water to that beaker. Describe what happens.
Part E: Effect of Temperature on the Equilibrium
1
Fill two test tubes about 2/3 full with some of the solution from Part B. Heat the solution in one test tube by placing the test tube in a beaker of hot water. Cool the solution in the other test tube by placing it in a beaker of ice water. The exact temperatures are not important.
2
Record your observations for the solutions in the hot bath and the ice bath, including the relative rates of the change seen in the test tubes at different temperatures.
3
Allow both solutions from Part E, Step 2 to come to room temperature. Record your observations.
Part F: Summary
Summarize the observations made on the [Co(H2O)6]2+/[CoCl4]2– equilibrium system for Parts A, C, D, and E (at both temperatures). This should include: the change seen in the color, what is being done to the system, the stress this places on the equilibrium (adding or removing reactant, product, or heat), and the interpretation of the direction the equilibrium shifts in response to the stress.| Part | What is Being Done | Change in Color | Type of Stress | Interpretation |
|---|---|---|---|---|
| A |
Addition of HCl
(Cl–) |
|||
| C |
Addition of
AgNO3 — Removes Cl– as AgCl |
|||
| D | Addition of Water | |||
| E-1 | Hot Bath | |||
| E-2 | Ice Bath |
