Computer Modeling of Molecules
Organization
-
•Pre-Lab: completion of Lewis structures at the end of lab 4
-
•Mode: inquiry, in pairs as needed
-
•Grading: lab notes and report
-
•Safety: none
Goal:
To use Lewis structures to predict the shape and molecular geometry of molecules.
To use Lewis structures to predict the shape and molecular geometry of molecules.
I: Background
Computer modeling and visualization of molecules using desktop computers and the web has become an important tool used by chemists and biochemists to increase their understanding of intra- and inter-molecular interactions. In this tutorial exercise, you will use the Lewis structures of molecules from Lab 4 and view them using a digital library to see their shapes and obtain physical data about the molecules—such as bond lengths, bond angles, and dipole moments. There will be computers available for your use, but you may choose to use your own computer. If you plan to use your own computer, please be certain that you have tested the software before coming to class. The background information on molecular structures can be found in the pre-lab material.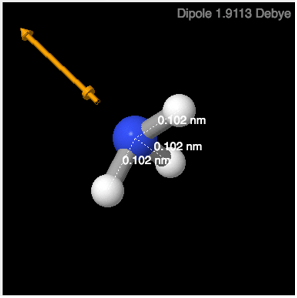
Figure 1: Ammonia, NH3
II: Exercises
Instructions
Please answer all questions in your laboratory notebook. Reproduce any structures and tables in your notebook as needed in order to answer the questions.Part A: Effect of Bond Order and Resonance on Bond Lengths
In this exercise, you will examine carbon-oxygen single and double bonds in six different molecules or ions—ethanol, dimethyl ether, acetic acid, acetaldehyde, acetone, and trifluoroacetate ion.
1
Collecting Data—www.chemeddl.org/resources/models360/models.php
-
a.Create a table to collect data on the carbon-oxygen bond lengths in the molecules. In the "Find" box, type the name of the molecule. By clicking the mouse in the molecule box, you can rotate the molecule. In the display window to the left of the molecule box, click "Bond Length". Rotate the molecule to display the C–O bonds.
2
Working With the Data—Students can work in groups of 2 to answer these questions.
-
a.What is the average C–O single bond length in ethanol, dimethyl ether, and acetic acid? Calculate the approximate variation in the C–O single bond length. To do this, calculate the difference in length between the average C–O bond length and the longest and shortest bond length listed in the table (calculate as a positive number). The variation is the average of these differences. Report your answer as average ± variation nm.
-
b.What is the average C
 O double bond length in acetone, acetaldehyde, and acetic acid, and what is the approximate variation in the C
O double bond length in acetone, acetaldehyde, and acetic acid, and what is the approximate variation in the C O double bond length? Report your answer as average ± variation nm.
O double bond length? Report your answer as average ± variation nm.
-
c.Based on the average values of the carbon-oxygen single and double bonds from questions a and b, can a single bond be distinguished from a double bond based on bond length? Why or why not?
-
d.Is the length of the carbon-oxygen bonds in the trifluoroacetate ion longer than or shorter than a typical carbon-oxygen single bond? Is the length of the carbon-oxygen bonds in the trifluoroacetate ion longer than or shorter than a typical carbon-oxygen double bond? Can the carbon-oxygen bonds be classified as either a single or a double bond based on length?
-
e.How would the bond length be reflected in the bond order for the carbon-oxygen bond in the trifluoroacetate ion? Estimate the bond order for the two carbon-oxygen bonds in the trifluoroacetate ion.
-
f.Refer to the resonance structures you previously drew for the trifluoroacetate ion. Does the bond length data collected here support the existence of the trifluoroacetate ion as a resonance hybrid of these structures? Explain your answer.
Part B: Geometry and Bond Angles—Effect of Lone Pairs
You will explore the effect that lone pairs on the central atom have on the bond angles in six different molecules—methane, ammonia, water, boron trifluoride, ozone, and ethyne.
1
Refer to the Lewis structures you previously drew for these six molecules. Create a table in your laboratory notebook that provides the following information for each of them: electron pair geometry (EPG), ideal bond angle for EPG, total number of electron pair regions (SN), number of bonding regions (CN), number of lone pair regions*, and the observed bond angle.
*A double or triple bond counts as one bonding region for the analysis of geometry.
2
Working With the Data—Students can work in groups of 2 to answer these questions.
A bond angle is the angle between the bonding pairs of electrons in a molecule. Bond angles reflect repulsive forces between all bonding pairs and lone pairs around the central atom in a molecule. The set of bonds will assume angles that minimize the total of these repulsive forces (VSEPR).
-
a.Consider the three molecules with no lone pairs on central atoms. How does the bond angle change with an increasing coordination number? Explain why this is so.
-
b.Consider the group of molecules with tetrahedral electron pair geometry. Explain how the presence of lone pairs changes the bond angles in a molecule—do bond angles increase or decrease as the number of lone pairs on the central atom increases? Is this same trend seen for the pair of molecules with trigonal planar electron pair geometry?
-
c.Think about the amount of space a lone pair requires compared to a bonding pair of electrons, which is restricted to the space between the atoms involved in the bond. Which would have a larger electron cloud, a lone pair or a bond pair of electrons? Which would exert a stronger repulsive force? Why?
-
d.Explain how the presence of lone pairs changes the bond angles in a molecule.
-
e.Explain why ozone has a larger bond angle than either water or ammonia.
Part C: Geometry and Dipole Moments—Effect of Atom Type and Number of Atoms
You will consider each of the following six molecules in this section—methane, chloromethane, bromomethane, dichloromethane, chloroform, and carbon tetrachloride. For each molecule, you will view three-dimensional displays of the electrostatic potential experienced by a point positive charge moving along a particular surface of the molecule, revealing variations in the electron density. You will also display the dipole moment vector of the molecule, if it has one. These visualizations will help you form conclusions about the relationship between the geometry of a molecule, the substitution pattern around the central atom, and whether or not the molecule is polar.
1
Create a table containing a row for each of the specified molecules. This table will be used to collect data.
-
a.Select "Molecular Dipole" to display the value of the dipole moment, μ (in units of Debye, D). Record this in the table. Sketch the orientation of the dipole moment on the Lewis structure in your notebook.
-
b.Go to the "Molecular Electrostatic Potential" menu. Select "MEP on van der Waals Surface" to see the electron density distribution over the molecule.
-
i.Describe the distribution of electron density over the molecule in the space provided in the table. Record your observations in the table.
-
ii.Does the electron density seem to be symmetrically distributed over the molecule, or is there a concentration of electron density (red color) over part of the molecule and a lack of electron density (blue color) over another part of the molecule?
-
iii.If not symmetrically distributed, where does the electron density seem to accumulate? Be detailed in your answer and reference a structural feature of the molecule, such as the location of a specific atom or bond or lone pair. Top and bottom are not good descriptions, because molecules tumble, so the "top" can be at the "bottom" or the "side" at any given moment.
-
-
c.Repeat the exercise for the remaining molecules.
2
Working With the Data
-
a.What is the electron pair geometry and the molecular geometry of the molecules in the table?
-
b.Compare methane with the four chlorinated methane molecules. What trends do you see between the number of chlorine atoms on the molecule and the dipole moment of the molecule? Why do you think this trend exists?
-
c.Do you think carbon tetrabromide or carbon tetraiodide will be polar or non-polar? Why?
-
d.Write a statement that you can use to determine when a tetrahedral molecule will be non-polar and when a tetrahedral molecule will be polar.
-
e.Compare chloromethane with bromomethane. How does the value of the dipole moment correlate with the relative electronegativity of chloride and bromine? Why do you think this trend exists?
Part D: Geometry and Dipole Moments—Effect of Lone Pairs
In Part C, you saw that tetrahedral molecules with four of the same type of atom connected to the central atom will always be non-polar. Like those tetrahedral molecules, methane, ammonia, and water have only one type of atom connected to the central atom. Will ammonia and water be non-polar, like methane, or polar? Or are there other factors, besides the types of atoms connected to the central atom, that influence whether or not a molecule is polar?
1
Create a table for all three molecules—methane, ammonia, and water. For each molecule, the table should contain the molecular geometry, the number of lone pairs on the central atom, the dipole moment in D, whether the molecule is polar or non-polar, and a description of the electron density of the molecule. The correspondence of the accumulation of negative charge with the relative electronegativity of the atoms in the molecule and the position of the lone pairs should be specifically addressed. Provide all of this information for all three molecules.
2
Working With the Data—Students can work in groups of 2 to answer these questions.
-
a.Does there seem to be a correlation between the presence of lone pairs on the central atom and the presence of a dipole moment?
-
b.Why do you think the presence of lone pairs creates a dipole moment in these molecules, even when all of the atoms connected to the central atom are the same type?
-
c.What relationship can you identify between the orientation of the dipole moment vector and the location of lone pair(s)?
-
d.Do you think ozone, O3, would be a polar molecule or a non-polar molecule? Explain why.
-
e.Write a statement about how you would evaluate a molecule with trigonal planar or tetrahedral electron pair geometry to determine if it is polar or not, taking into account both Parts C and D. What is the first thing you need to do to evaluate if a molecule is polar or not?
