Solutions and Attractive Forces
Organization
-
•Mode: inquiry, groups of 2
-
•Grading: lab notes and post-lab report
-
•Safety: lab goggles; latex or nitrile gloves recommended
Goal:
In this lab, you will evaluate structures of substances to determine the sorts of intermolecular forces between those substances, predict the sorts of intermolecular forces that form between substances of different types, observer solubility behavior, and come to an understanding of the concept of "like dissolves like".
In this lab, you will evaluate structures of substances to determine the sorts of intermolecular forces between those substances, predict the sorts of intermolecular forces that form between substances of different types, observer solubility behavior, and come to an understanding of the concept of "like dissolves like".
| CHEM 115 Expt. 6 | Chemical Classification | Possibility Of: | NFPA Codes | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intermolecular Forces |
Poison A
|
Flammable Gas
|
Flammable Liquid
|
Combustable Liquid
|
Reacts with Water
|
Oxidizer
|
Organic Peroxide
|
Poison B
|
Corrosive Acid
|
Corrosive Base
|
Irritating or Harmful
|
Misc. Hazard
|
No Hazard
|
Fire
|
Sudden Release of Pressure
|
Reactive
|
Immediate (Acute) Health Hazard
|
Delayed (Chronic) Health Hazard
|
Fire
|
Health
|
Reactivity
|
Special Precautions
|
| Hexane | X | X | X | X | 3 | 1 | 0 | |||||||||||||||
| Methanol | X | X | X | 3 | 1 | 0 | ||||||||||||||||
| Ethanol | X | X | X | 3 | 1 | 0 | ||||||||||||||||
| 1-Propanol | X | X | X | 3 | 1 | 0 | ||||||||||||||||
| 1-Butanol | X | X | X | 3 | 1 | 0 | ||||||||||||||||
| 1-Hexanol | X | X | X | 3 | 2 | 0 | ||||||||||||||||
| Methyl Orange | X | X | 0 | 2 | 0 | |||||||||||||||||
| Iodide | X | X | X | 0 | 3 | 1 | ||||||||||||||||
| Potassium Nitrate | X | X | X | 0 | 1 | 0 | ||||||||||||||||
| Sucrose | X | 1 | 1 | 0 | ||||||||||||||||||
I: Background
The figure below shows the different types of intermolecular forces that can exist between different types of molecules. It will be up to you to decide which types exist (and how important they are) for the different solutions explored in this lab.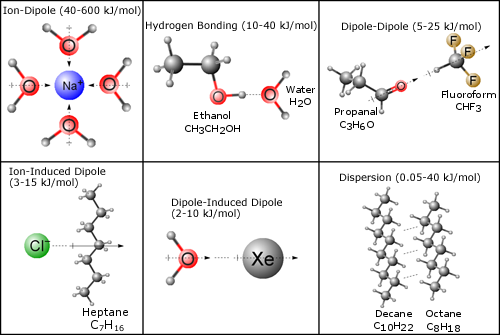
Figure 1: Intermolecular Forces Between Different Types of Molecules
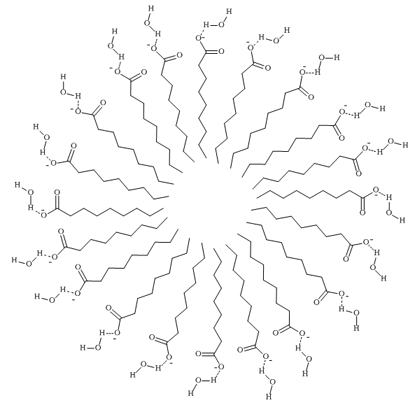
Figure 2: Soap Micelles
II: Exercises
Answer all questions in your laboratory notebook. You do not have to reproduce the questions.Part A: Demonstration
Two burets have been set up, one containing cyclohexane and the other containing distilled water. Information about these two substances is given in Table 1. Classify each molecule as polar or non-polar.| Water | Cyclohexane | |
|---|---|---|
| Formula | H2O | C6H12 |
| Lewis Structure | 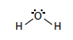 |
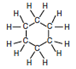 |
| Dipole Moment (debye, D) | 1.86 | 0 |
-
1.What sorts of intermolecular forces are there between two molecules of water?
-
2.What sorts of intermolecular forces are there between two molecules of cyclohexane?
-
3.Your instructor will conduct a demonstration of how a stream of water molecules and a stream of cyclohexane molecules interact with a charged surface.
-
a.When the charged rod was held next to the stream of water, what did you observe?
-
b.When the charged rod was held next to the stream of cyclohexane, what did you observe?
-
c.Which molecule is deflected by the charged rod—the polar or the non-polar molecule?
-
d.For the molecule deflected by the charged rod, explain what you think is going on between the rod and the molecules to create the effect you observed.
-
Part B: Getting Ready for Experiments
Get a clean, dry 50 mL beaker. Pour 10 mL of hexane into the beaker—you can use the volume markers on the side of the beaker. This is all the hexane you will have to work with for both Parts C and D. Label the beaker. Put about 20 mL of distilled water into another clean, dry 50 mL beaker with a label. Get several small, clean, dry test tubes of the same size, and several clean, dry plastic measuring pipets. Be prepared to label everything.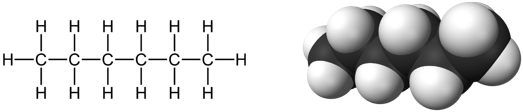
Figure 3: Two Views of Hexane, CH3CH2CH2CH2CH2CH3
Part C: Interactions of Solid Solutes With Different Solvents
Read Part B before starting.
1
Set up and label six test tubes. Using a clean spatula for each solid, place a small chunk of iodine (this size:  ) in two test tubes. Place a tiny dab of Methyl Orange (this much:
) in two test tubes. Place a tiny dab of Methyl Orange (this much:  ) in two test tubes, and a small scoop of potassium nitrate (about twice as much as the other two solids) in the last two test tubes.
) in two test tubes, and a small scoop of potassium nitrate (about twice as much as the other two solids) in the last two test tubes.
2
Use a plastic measuring pipet to add 1.0 mL of distilled water to one of the test tubes with iodine, Methyl Orange, and potassium nitrate. Shake or flick the test tubes gently, and record your observations in the table in your notebook.
3
Use another plastic measuring pipet to add 1.0 mL of hexane to the other test tubes with the solids. Shake or flick the test tubes gently and then let them settle for a bit, and record your observations in the table in your notebook.
4
Discuss the solubility of the three solids in the polar solvent and in the non-polar solvent. For each solid, can you conclude that the solid is more soluble in the polar solvent or in the non-polar solvent? Describe how you decided this, and back up your decision with your observations.
5
Carefully pour the contents of the test tube with water and iodine into the test tube with hexane and iodine. Shake the test tube for a bit to allow thorough mixing and complete dissolution, and then let the contents sit for a minute. Describe everything you observe.
-
a.Do you see one layer of liquid or more than one layer of liquid? If you see more than one layer of liquid, what is the identity of each layer? What evidence are you using to make the identification? Draw a picture of the test tube, labeling the contents of the layer you see.
-
b.Based on what you observed when you mixed the two solutions together, in which solvent is iodine more soluble?
6
Combine the test tubes containing Methyl Orange. What do you observe? Can you conclude in which solvent Methyl Orange is more soluble?
7
Combine the test tubes containing potassium nitrate. What do you observe? Can you conclude in which solvent potassium nitrate is more soluble?
Working With Data
1
What sorts of intermolecular forces are there between two molecules of water? What sorts of intermolecular forces are there between two molecules of hexane?
2
Refer to the structure of Methyl Orange. Carbons are located at all unlabeled intersections and have hydrogens attached. There is an ionic group (the sodium sulfite-like group at right) attached to an organic group. Looking at the organic part of the molecule, speculate if you think that part of the molecule is polar or non-polar (or a little bit of both).
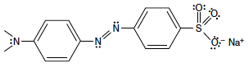
Figure 4: Methyl Orange
3
Classify each of the solutes (iodine (I2), Methyl Orange, and potassium nitrate) as polar, non-polar, or ionic (more than one classification may apply for Methyl Orange).
4
What sorts of intermolecular forces exist between one molecule of I2 with another molecule of I2? What sorts of intermolecular forces exist between molecules of Methyl Orange? What sort of force holds the potassium cation and the nitrate anion together?
5
Create a table with the same columns and rows as before. For each solute, place an X in the box under the solvent in which the solute is most soluble, and write in that box the types of intermolecular forces expected between the solute and the solvent particles.
-
a.Are the solute-solvent intermolecular forces in the box with an X similar or dissimilar to the types of intermolecular forces between two solute molecules?
-
b.Are the solute-solvent intermolecular forces similar in the boxes with an X or dissimilar to the types of intermolecular forces between two solvent molecules?
6
The potential solubility of a solute in a solvent is predicted using the concept of "like dissolves like", which you observed during this exercise. Write a statement describing what "like dissolves like" means.
Making Predictions
The structure of sucrose (sugar) is given in Figure 5. (The unlabeled intersections are carbon atoms.)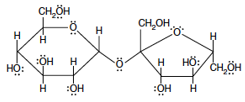
Figure 5: Sucrose
1
Predict the relative solubility of sugar in water and in hexane. Explain your reasoning.
2
Test your prediction. Summarize your test in your notebook. If your prediction was not correct, evaluate the structure again to explain the results of the solubility test.
Caution:
Hazardous Waste—Dispose of the contents of the test tubes in the hazardous waste bottle in the hood.
Hazardous Waste—Dispose of the contents of the test tubes in the hazardous waste bottle in the hood.
Part D: The Effect of Carbon Chain Length on the Solubility of Alcohols in Different Solvents
Set up a table for your observations in your laboratory notebook.1
Set up and label 10 test tubes. Using a different clean, graduated plastic pipet, put 1.0 mL of each alcohol in two test tubes (two test tubes with methanol, two test tubes with ethanol, etc.).
If methanol is available in a squeeze bottle, squeeze the methanol into the test tube directly, estimating the volume by comparison with the volume of alcohol in the other test tubes.
2
Use a plastic measuring pipet to add 1.0 mL of distilled water to one of the test tubes with methanol, one of the test tubes with ethanol, one of the test tubes with 1-propanol, one of the test tubes with 1-butanol, and one of the test tubes with 1-hexanol. Shake or flick the test tubes gently, and then let the mixtures settle. Record your observations in the table in your notebook. Be specific in your observations, recording relative volumes of any layers you observe in the test tube. If you see layers, are the layers equal in volume?
3
Add 1.0 mL of hexane to each of the other test tubes with the alcohols. Shake or flick the test tubes gently, and then let the mixtures settle. Record your observations in the table in your notebook. Be specific in your observations, recording relative volumes of any layers you observe or the volume in the test tube. If you see layers, are the layers equal in volume?
4
Discuss the solubility of the different alcohols in the polar solvent and in the non-polar solvent. For each alcohol in turn, is the alcohol more soluble in the polar solvent or in the non-polar solvent? Describe how you decided this, or if you can decide this or not.
5
Carefully pour the test tube containing hexane and methanol into the test tube containing water and methanol. What do you observe? Be specific in your observations, noting changes in the layers or volumes of layers that you observe.
-
a.Do you observe one layer of solution or more than one layer? Draw a picture of the test tube with contents showing correct relative proportions of any layers of liquids seen. Label the layers.
-
b.In which solvent is methanol more soluble, the polar solvent or the non-polar solvent?
6
Repeat Step 5 for each of the other sets of alcohol in hexane and water, describing what you observe, the contents of each layer, and determining in which solvent the alcohol is more soluble.
Working With Data
-
1.What sorts of intermolecular forces exist between molecules of water? What sorts of intermolecular forces exist between molecules of hexane?
-
2.Draw the Lewis structure for each alcohol (formulas are given below). What sorts of intermolecular forces are there between a molecule of methanol with another molecule of methanol? Do this analysis for each alcohol.
| methanol | ethanol | 1-propanol | 1-butanol | 1-hexanol |
|---|---|---|---|---|
| CH3OH | CH3CH2OH | CH3CH2CH2OH | CH3CH2CH2CH2OH | CH3CH2CH2CH2CH2CH2OH |
-
3.What sorts of intermolecular forces exist between an alcohol and a water molecule? What sorts of intermolecular forces exist between an alcohol and a hexane molecule?
-
4.How do the dispersion forces change as the length of the hydrocarbon chain increases for the series of alcohols? Why do they change?
-
5.As the length of the hydrocarbon chain increased in the series of alcohols, did the solubility of the alcohol in water increase or decrease?
-
6.As the length of the hydrocarbon chain increased in the series of alcohols, did the solubility of the alcohol in hexane increase or decrease?
-
7.Which intermolecular force do you think determined the solubility of the alcohols in hexane as the length of the hydrocarbon chain increased? Explain your answer.
Caution:
Hazardous waste—dispose of the contents of the test tubes in the hazardous waste bottle in the hood.
Hazardous waste—dispose of the contents of the test tubes in the hazardous waste bottle in the hood.
