Introduction to Chemical Reactions
Organization
-
•Mode: inquiry, groups of 2, and individual work
-
•Grading: lab notes and post-lab report
-
•Safety: goggles, closed-toe shoes, long pants/skirt/sleeves required, latex or nitrile gloves are recommended
Goal:
Learn how to use patterns of reactivity of ions to determine the identity of an unknown ion.
Learn how to use patterns of reactivity of ions to determine the identity of an unknown ion.
| CHEM 115 Expt. 7 | Chemical Classification | Possibility Of: | NFPA Codes | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Introduction to Chemical Reactions |
Poison A
|
Flammable Gas
|
Flammable Liquid
|
Combustable Liquid
|
Reacts with Water
|
Oxidizer
|
Organic Peroxide
|
Poison B
|
Corrosive Acid
|
Corrosive Base
|
Irritating or Harmful
|
Misc. Hazard
|
No Hazard
|
Fire
|
Sudden Release of Pressure
|
Reactive
|
Immediate (Acute) Health Hazard
|
Delayed (Chronic) Health Hazard
|
Fire
|
Health
|
Reactivity
|
Special Precautions
|
| Hydrochloric Acid Solution | X | X | X | 0 | 3 | 2 | COR | |||||||||||||||
| Sulfuric Acid Solution | X | X | X | 0 | 2 | 1 | ||||||||||||||||
| Ammonium Hydroxide Solution | X | X | X | 1 | 3 | 2 | COR | |||||||||||||||
| Strontium Nitrate Solution | X | 0 | 1 | 1 | ||||||||||||||||||
| Lead(II) Nitrate Solution | X | X | X | X | 0 | 3 | 2 | |||||||||||||||
| Zinc Nitrate Solution | X | X | 0 | 2 | 1 | COR | ||||||||||||||||
I: Background
An important part of becoming a scientist is developing good observation skills and learning how to use observations in combination with deductive reasoning to draw conclusions about events in the physical world. For chemists this often means devising a contrived scheme, called an experiment, which can be used in the laboratory to investigate a particular question, referred to as a hypothesis. If the experiment involves the mixing together of two or more substances, then careful observation can determine if any new substances formed, how long it took for the new substances to form, and how much energy was transferred as heat. Careful measurements of the quantities of the substances and the energy transferred during the reaction are also important observations. Through the use of deductive reasoning based on these observations, conclusions can then be drawn about the chemical behavior of matter. Deductive reasoning also results in other questions that need to be answered, and this leads to further experiments. Eventually, an analysis of all the conclusions furthers our overall understanding of the nature of matter and its behavior under all kinds of conditions. This laboratory will also give you an introduction into the names, formulas, and reactivity of compounds you will encounter throughout the rest of the course. Laying a strong foundation in this material at the beginning of the semester will increase your chances of success later on. Refer to the Appendices for valuable information on many of the ions and ionic compunds you'll be dealing with in this lab, including a Table of Solubilities, which will help you make sense of your results. In the exercise, you will make a series of observations about the way different cations react with reagent solutions of acids and bases. The pattern of reactivity will be used to develop a scheme by which you would be able to identify an unknown cation based on its reactivity. Good observations and deductive reasoning skills are needed to successfully carry out this exercise. You will be graded on the quality of your lab notes for this exercise, as well as your success in identifying your unknown. Read the section on keeping a laboratory notebook in the introduction and follow those guidelines.II: Exercises
Part A: Observing the Pattern of Reactivity for Different Cations in Solution
Experimental Procedure
Each group of two students will do all of the tests for all of the solutions and assemble a complete table of data. Please read and follow the instructions carefully. In many cases you need only to perform tests on those solutions in which a precipitate formed, so pay attention to the instructions to save time and reagents. You will be testing three solutions, each of which contains a different cation dissolved in water. The cations you will be studying are strontium ion, Sr2+; lead(II) ion, Pb2+; and zinc ion, Zn2+. You will be comparing the reactions that take place between the cations and two different acids or a base—hydrochloric acid, sulfuric acid, and ammonium hydroxide. To be sure that the chemical behavior observed with the reagent is due to the presence of the cation in solution, it is important to have an experimental control. Discuss with your lab partner what you think an appropriate experimental control would be for this exercise, and confirm with your lab instructor before starting the experiment.Getting Started
-
•Clean and label a test tube for each cation solution (Sr2+, Pb2+, and Zn2+) plus one control.
-
•Clean and label an additional 3 test tubes, one for each of the acids and base (HCl, H2SO4, and NH4OH). Fill each test tube about half-way with the appropriate reagent solution from the squeeze bottle.
-
•Rinse about 6 graduated plastic pipets thoroughly in distilled water.
-
•Rinse one 24-well microplate. Note the labels on the top and left side of the plate are used for identification of samples.
-
•Get a large beaker for the bench, labeled "WASTE". Collect your test tube waste here as you go.
Caution:
PAY ATTENTION to the number of drops you are instructed to add. Results may vary if you add too much or not enough reagent at each step. Practice using the pipets to deliver drops—make sure you add a full drop of solution, and not a bubble of solution.
PAY ATTENTION to the number of drops you are instructed to add. Results may vary if you add too much or not enough reagent at each step. Practice using the pipets to deliver drops—make sure you add a full drop of solution, and not a bubble of solution.
Acid/Base Tests
1
Place 1 mL of each cation solution and the control into microplate wells. Note the cell number (e.g. A1 or B4), and keep a record of which tests are in each well.
2
Primary Tests—Using a clean graduated plastic pipet, add 1 drop of the acid or base solutions to each of the cation test tubes and the control. After each drop, carefully mix the solution by gently swirling or agitating the plate. You should not need to add more than 5 drops.
Watch for any reaction. Record your observations in your laboratory notebook. These notes should be detailed. Do not simply record that "a precipitate formed", but do record, for example, that "copious quantities of slightly yellow, very small particle-sized precipitate formed."
3
Secondary Tests—ONLY in wells where a precipitate formed, add 5 to 6 more drops of the acid or base solution and mix. Record your observations in your laboratory notebook.
4
Discard the contents of the cation solution test tubes and control into the waste beaker on your bench. Use your distilled water squeeze bottle from the locker to rinse solid and chemical residue from the test tubes into the waste beaker. Rinse the test tubes with more distilled water, and shake out any excess water.
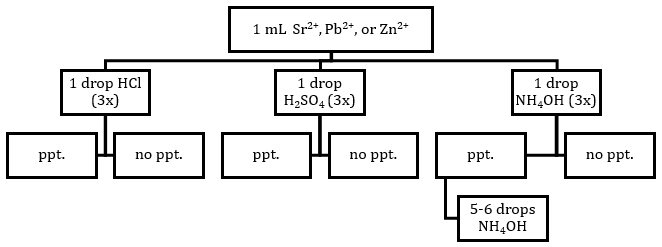
Figure 1: Flow Chart for Acid/Base Tests of Cations
Data Summary
When the three reagents are dissolved in water they dissociate into an anion and a cation. The dissociation reactions are as follows.
HCl → H+ + Cl–
H2SO4 → 2 H+ + SO42–
NH4OH → NH4+ + OH–
H2SO4 → 2 H+ + SO42–
NH4OH → NH4+ + OH–
Part B: Analysis of Unknown Solutions
You will now use the results in Table 1 to devise an analysis scheme that can be used to identify the solutions of cations (Sr2+, Pb2+, or Zn2+) in water. The first step is to prepare an analysis scheme in the form of a flow chart that describes the series of tests using HCl, NH4OH, or H2SO4 that could be used to identify any of the cations Sr2+, Pb2+, and Zn2+ in a solution. The end goal is to have a flowchart with arrows pointing to other tests that ends in 3 boxes that contain only one cation. The result of each test points the way to the next test you need to perform.-
1.The first test has been selected for you. Look at the data and deduce which cations are present if the HCl test results in a precipitate, and which cations are present if the HCl test does not result in a precipitate. Recreate this flow chart in your notes, and fill in the missing information in the boxes in your notes.
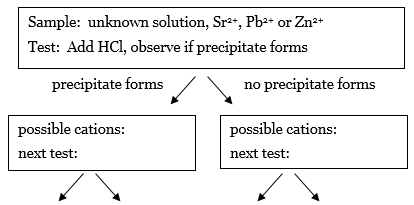
Figure 2
-
2.For the cations in the box on the left, which reagent could be used to distinguish between the cations? Write the name of the next test in the box, the possible outcomes by each arrow, and the conclusion about the cation(s) that could be drawn for each outcome.
-
3.Repeat this process for the box on the right.
-
4.If the next row of boxes has two or more cations in the same box, go through the process again until each box at the end of an arrow has only one cation present.
Part C: Analysis of Unknown
You are going to be given an unknown, which will contain one of the cations, or distilled water. Based on the observed reactions between your unknown and the acid or base reagents, you should be able to deduce the identity if your unknown solution contains Sr2+, Pb2+, or Zn2+, or deduce that there is no cation present using your scheme from Part B.Caution:
Each student will be given an unknown solution to work up by themselves as individuals. Be sure to record the sample number of your unknown in your laboratory notebook.
Each student will be given an unknown solution to work up by themselves as individuals. Be sure to record the sample number of your unknown in your laboratory notebook.
Caution:
For each new test, start with a fresh sample of unknown in a clean microplate well. For example, don't add H2SO4 to a well in which you've already added HCl—you will get erroneous results.
For each new test, start with a fresh sample of unknown in a clean microplate well. For example, don't add H2SO4 to a well in which you've already added HCl—you will get erroneous results.
-
•the test you are doing
-
•the number of drops of reagent added
-
•what you observe after adding the reagent
-
•what the results of the test means
-
•the next test to carry out
