Lab Investigation 2 - How Much Acetic Acid is in Vinegar?
Guiding Question
What factors determine how accurate and how precise the concentration of acetic acid in vinegar can be determined?Introduction
Many laboratories analyze consumer products to determine accuracy in the labeling of the product. The very common and simple technique of titration is demonstrated in this experiment. A titration is an analytical procedure in which a reaction is run under carefully controlled conditions. The stoichiometric volume of one reactant of known concentration, the titrant, that is required to react with another reactant of unknown concentration, the analyte, is measured. The concentration of the analyte is determined from the concentration and volume of titrant and the stoichiometry of the reaction between them. The experimental setup is shown in Figure 1. A buret, which contains the titrant, is calibrated so the volume of solution that it delivers can be determined with high accuracy and precision. Titrant is added to the analyte until the stoichiometric volume of titrant has been added. This is called the equivalence point, at which the volume of titrant delivered by the buret is read. Usually, the volume readings are estimated to the nearest 0.01 mL. The delivery of the titrant is adjusted with the stopcock on the buret. With practice, one can dispense fractions of a drop of titrant and control the procedure well enough that replicated titrations agree within 0.10 mL. For this first lab, you will need your titrations to agree to within 0.50 mL.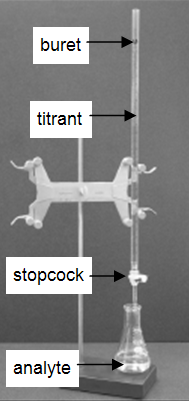
Figure 1: Titration setup
( 1 )
HC2H3O2(aq) + OH−(aq) → C2H3O2−(aq) + H2O(l).
( 2 )
moles HC2H3O2 reacting = moles OH− added.
( 3 )
moles of acid reacting = moles of base reacting.
(molesbase = Mbase × Vbase).
As a reminder on concentration units, molarity is defined as the number of moles of solute in a liter of solution (M = mol/L). This is numerically equal to the number of millimoles of solute in a milliliter of solution (M = mmol/mL). It is often convenient to use this second definition of molarity in titrations and other work where small quantities are involved. There are 1000 mmol in 1 mol and 1000 mL in 1 liter.
For example, 10.2 mL of 0.100 M NaOH solution contains 1.02 mmol of NaOH.
( 4 )
10.2 mL solution ×
= 1.02 mmol NaOH
| 0.100 mmol NaOH |
| 1 mol solution |
(Macid = molesacid/Vacid).
In this experiment, a carefully measured volume of vinegar (Vacid)
is placed into a flask and the mass determined. The sample of vinegar is then titrated with a NaOH solution of known concentration (Mbase),
and the volume of NaOH solution required to reach the endpoint (Vbase)
is determined. Vbase,
Mbase,
and Vacid
are all known, so the concentration of the acid (Macid)
can be determined as described above. In addition, the mass of acetic acid in the sample can be determined from the number of moles present and the molar mass of acetic acid (gacid = MWacid × molesacid).
Finally, the mass percent of acetic acid in the vinegar can be determined from the mass of the acetic acid in the sample and mass of the vinegar solution that was titrated.
( 5 )
mass % =
× 100
| mass of acetic acid in sample |
| mass of vinegar solution titrated |
Goals
As you complete this investigation you will:-
1Standardize a NaOH(aq) solution.
-
2Titrate a vinegar sample with the standardized NaOH(aq) solution.
-
3Measure the density of a vinegar sample.
-
4Calculate the molarity and mass percent of acetic acid in the vinegar sample.
Materials Available for Use
- Vinegar
- NaOH(aq) solution
- KHP - potassium hydrogen phthalate
- Phenolphthalein indicator
- 10-mL pipets
- Beakers
- Erlenmeyer flasks, 125 mL
- Buret stand with buret
- Funnel
- Analytical balances
Safety Precautions
Caution:
NaOH is corrosive. It can attack the skin and cause permanent damage to the eyes. If NaOH solution splashes into your eyes, use the eyewash station immediately. Hold your eyes open and flush with water. If contact with skin or clothing occurs, flush the affected area with water. Have your lab partner notify your instructor about the spill.
NaOH is corrosive. It can attack the skin and cause permanent damage to the eyes. If NaOH solution splashes into your eyes, use the eyewash station immediately. Hold your eyes open and flush with water. If contact with skin or clothing occurs, flush the affected area with water. Have your lab partner notify your instructor about the spill.
Getting Started
Your first task is to standardize the NaOH solution using solid KHP (KC8H5O4, 204.22 g/mol). This means you need to determine its molarity to at least three significant figures. You will need at least three titrations that agree within 1% as described in the procedure. You have two other tasks to accomplish in the lab. You must determine the density of the vinegar solution and the molarity of the acetic acid in vinegar. The mass percent of acetic acid can be calculated from your data. To accomplish your tasks, you will need to make very accurate volume and mass measurements. Burets and pipets are useful in accurately measuring volumes. The buret and pipet are described in the introduction and videos. Before you begin, make sure you understand their proper use.Procedure
Please print the worksheet for this lab. You will need this sheet to record your data.Preparation of Buret
1
Check the buret by rinsing down the sides with distilled water bottle to check if water "sheets" down inside of the buret. If water droplets are observed, the buret should be washed before use. Be careful not to scratch the inner surface if you find it necessary to use a buret brush to clean it. Rinse the buret well with tap water, including the stopcock and washers. Then rinse down the walls of the buret with deionized/distilled water.
2
Finally rinse the buret at least TWICE with small portions of your NaOH solution to ensure that all water is removed. Run the solution out through the tip.
3
Fill the buret with NaOH solution using a funnel.
4
To REMOVE TRAPPED AIR BUBBLES in the tip of the buret after filling, QUICKLY OPEN AND CLOSE THE STOPCOCK SEVERAL TIMES. Note: If the stopcock lines up correctly with the buret tip, the trapped bubbles are not as prevalent. Check with the instructor if the bubble persists.
Standardization with KHP
1
Use ~1 gram of KHP. IMMEDIATELY RECORD THE MASS IN THE DATA CHART.
2
Use the distilled water wash bottle to ensure that all the samples have been transferred into the flask.
3
Add about 50 mL of distilled water and two or three drops of indicator. The indicator phenolphthalein will be used in this titration experiment. Do not use too much indicator or you will be titrating that molecule as well. Be consistent in using the same number of drops in all the samples.
Titration Technique
1
Place a sheet of white paper beneath the receiving flask to more easily observe the endpoint.
2
Use a split white top/black bottom card to aid in reading the meniscus. Placing the card behind the buret and the black line just below the meniscus darkens the meniscus for easy reading. RECORD THE INITIAL VOLUME OF NaOH SOLUTION TO THE NEAREST HUNDREDTH MILLILITER (±0.01 mL) IN THE DATA TABLE OF YOUR NOTEBOOK.
3
The titrant (NaOH solution in buret) can be added fairly quickly at first, but as the endpoint is approached, the rate of addition should be slowed. If you are right-handed it is faster to add the titrant with your left hand while swirling with your right (vice versa for left-handers). As the endpoint is approached, the pink color will persist longer and longer. Near the endpoint, rinse the flask walls down with distilled water to ensure that all the added NaOH base has reacted.
4
When very close to the endpoint, suspend a half-drop of base on the tip of the buret and rinse the drop into the receiving flask with the distilled water wash bottle. Another method used to add a fraction of a drop is to rotate the stopcock 180 degrees very quickly; however, you can inadvertently add too much base if you do this technique incorrectly.
5
The endpoint occurs when the phenolphthalein changes from clear to the faintest pink color you can see and persists for a minimum of 30 seconds. RECORD THE FINAL VOLUME OF NaOH SOLUTION TO THE NEAREST HUNDREDTH MILLILITER (±0.01 mL) IN THE DATA TABLE. Then determine amount of NaOH solution used in the titration.
6
Your group must complete a MINIMUM of FOUR TRIALS and three trials must agree WITHIN ±1%. You may actually do more depending upon your technique. YOU CANNOT JUST "CROSS OUT" DATA BECAUSE YOU DON'T LIKE IT; YOU MUST INDICATE THE REASON FOR DISCARDING. The reason may be obvious such as "overran endpoint" or you may only be able to discard value based upon statistical tests.
Molarity of Acetic Acid in Vinegar
1
Condition the buret with standardized NaOH from the previous week.
2
Condition a 10-mL pipet with the vinegar solution.
3
Measure 10 mL of vinegar into an Erlenmeyer flask and add phenolphthalein indicator.
4
Titrate with the standard NaOH from the previous lab.
Interactive Poster Session
Once your group has completed your work, prepare a whiteboard that you can use to share and justify your ideas. See the handout provided for details on this process.Report
Once you have completed your research, you will need to prepare an investigation report that consists of three sections. This report may require more than two pages with data tables. This report must be typed and any diagrams, figures, or tables should be embedded into the document.- Section 1: What concept and/or technique were you investigating, and how does it relate to the guiding question? What factors determine how accurate and how precise the concentration of acetic acid in vinegar can be determined? Describe titration and its use in conjunction with molar stoichiometry to determine concentration.
- Section 2: How did you go about your work, and why did you conduct your investigation in this way? Specifically, what measures did you take to insure accuracy and precision?
- Section 3: The argument in this investigation is not so much for your result but for the validity and reliability of your data. This report should include a comprehensive data table similar to the one you used in the pre-lab exercise where you found the molarity of your base. Do not report on the base standardization process, other than to report the NaOH molarity with standard deviation. You need to provide justification for discarding any of the vinegar titration trials. You should show one complete calculation of the molarity and mass % of the vinegar. Your final answer for the vinegar molarity should include a standard deviation.
- This third section is where you not only present your data, but use the values you obtain as evidence in your reasoning. Statements like, "see data table for values" are not acceptable!
Remember: An argument is not just an answer to the question. It is a claim or conclusion supported by evidence with a rationale for why the evidence supports the claim or conclusion.

