Ionic Radii
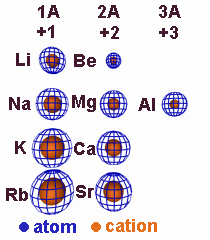
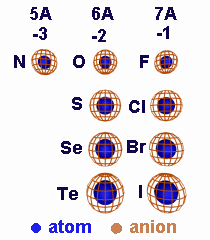
| Li | 1.52 | Be | 1.11 | N | 0.75 | O | 0.73 | F | 0.71 | ||
| Li1+ | 0.60 | Be2+ | 0.31 | N3– | 1.71 | O2– | 1.40 | F1– | 1.36 | ||
| Na | 1.86 | Mg | 1.60 | Al | 1.43 | S | 1.04 | Cl | 0.99 | ||
| Na1+ | 0.95 | Mg2+ | 0.65 | Al3+ | 0.50 | S2– | 1.84 | Cl1– | 1.81 | ||
| K | 2.27 | Ca | 1.97 | Se | 1.17 | Br | 1.14 | ||||
| K1+ | 1.33 | Ca2+ | 0.99 | Se2– | 1.98 | Br1– | 1.95 | ||||
| Rb | 2.48 | Sr | 2.15 | Te | 1.37 | I | 1.33 | ||||
| Rb1+ | 1.48 | Sr2+ | 1.13 | Te2– | 2.21 | I1– | 2.16 |
Atomic and Ionic Radii (Å) of Selected Main Group Elements
