Lab Investigation 3 - What is the identity of the unknown hydrate?
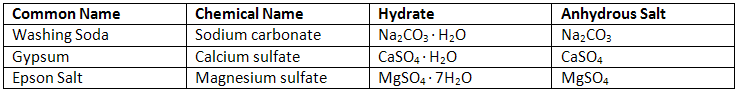
Identification of Hydrates Based on Percent Water
Guiding Question
What is the identity of the unknown hydrate?Introduction
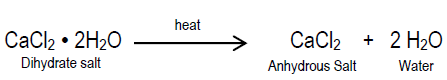
When an ionic compound (such as salt) is crystallized from an aqueous solution (such as salt water), the resulting solid crystals may appear to be perfectly dry. When the crystals are heated, however, the mass of the solid may decrease as water is released from the crystal structure. The form or appearance of the crystals may change and, in some cases, the color of the crystals may also change. Compounds that contain water molecules as part of their crystal structure are called hydrates. Are hydrates pure substances or are they simply "wet salts," that is mixtures containing variable amounts of water? A hydrate is a pure substance because it contains water molecules embedded in its crystal structure that do not vary. Heating a hydrate "drives off" the water molecules, and the solid that is left behind is called anhydrous (which means "without water"). The chemical formula of hydrate specifies the relative number of each kind of atom in a molecule, as well as the number of water molecules bound to each molecule. Calcium chloride dihydrate (road salt) is an example of a hydrate. The chemical formula for calcium chloride dihydrate is CaCl2 · 2 H2O. The "dot" in the chemical formula indicates that two water molecules (H2O) are attached or bound to the calcium chloride (CaCl2) ions by weak chemical bonds. The water molecules in calcium chloride dehydrate can be removed by heating the hydrate (see below).The Problem
You will be given an unknown hydrate and asked to identify this hydrate from a list of possible unknowns by determining the percent water in the hydrate.Materials available for use
- Test tubes
- Test Tube clamp
- Beaker
- Top Loader Electronic Balances
- Bunsen Burner
- 0.5 to 1.0 gram of unknown hydrate
Safety Precautions
Caution:
Never point the heating test tube at a person.
Never point the heating test tube at a person.
Caution:
Only heat to temperature needed to remove water, excess heat will result in oxidation of the salt.
Only heat to temperature needed to remove water, excess heat will result in oxidation of the salt.
Caution:
Wear goggles at all times.
Wear goggles at all times.
The Bunsen Burner
Getting Started
The unknown hydrate may be any of the compounds in the table on the design page. To determine the amount of water found in a hydrate experimentally, you must remove the water from the hydrate. To do this, you will need to heat the hydrate with a flame in order to evaporate the water (see Figure 1).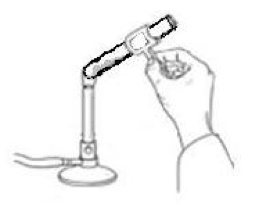
Figure 1: How to evaporate water from a hydrate.
Hydrate Investigation Design
Before you begin you will need to design an experiment for each unknown that includes alternative hypotheses, the test, and predictions. Download the worksheet and fill in the table and flow chart.Procedure
1
Be sure to weigh your empty test tube, so you do not lose material transferring between containers.
2
Stand the test tube in the beaker after zeroing the balance with the beaker on the pan.
3
Use 0.5 to 1.0 g of your unknown.
4
Spread the salt up the side of the test tube to increase surface area.
Interactive Poster Session
Once your group has completed your work, prepare a whiteboard that you can use to share and justify your ideas. See the handout provided for details on this process.Report
Once you have completed your research, you will need to prepare an investigation report that consists of three sections. Each section should provide an answer for the following questions. Your report should answer these questions in 2 pages or less. This report must be typed and any diagrams, figures, or tables should be embedded into the document. Generally, you need one page for the first two sections and the second page for the third section. The third section is where you not only present your data but use the values you obtain as evidence in your reasoning. Be sure to write in a persuasive style; you are trying to convince others that your explanation is acceptable or valid! Statements like, "see data table for values" are not acceptable!- Section 1: What concept were you investigating, and how does it relate to the guiding question? What is the identity of the unknown hydrate? See the introduction on hydrates to get started. You should discuss what a hydrate is and how you can identify a hydrate using the Law of Constant Proportions.
- Section 2: How did you go about your work and why? This is NOT the details of your procedure, but discussion of the processes. For example, describe the methods evaporating water from the hydrate efficiently.
- Section 3: What is your argument? There should be a data table with mass values from before and after heating. Discuss the validity and reliability of your data in answering the question. Make clear your reasoning from percent water to identity of the hydrate. You can directly compare your results with the group that had the same unknown.
- This third section is where you not only present your data, but also use the values you obtain as evidence in your reasoning. Statements like, "see data table for values" are not acceptable!