Chapter 4 – Ionic Bond
Introduction
Atoms can gain or lose valence electrons to become ions. Ions can be monatomic, such as Ca2+ and Cl1–, or polyatomic, such as NH41+ and CO32–. An ionic bond is the electrostatic (Coulombic) force of attraction between two oppositely charged ions. Ions and how they bond are the topic of this chapter.4.1 Ionic Bonding
Introduction
Ionic bonds are the electrostatic attraction of oppositely charged ions. The cation is usually a metal, and the anion is usually a nonmetal.4.1-1. Introduction to Bonding Video
- Viewing the Video
-
•View the video in this window by selecting the play button.
-
•Use the video controls to view the video in full screen.
-
•View the video in text format by scrolling down.
-
•Jump to the exercises for this topic.
4.1-2. Ionic Bonding
An ionic bond is the Coulombic attraction of two oppositely charged ions.
Compounds between metals and nonmetals are ionic.
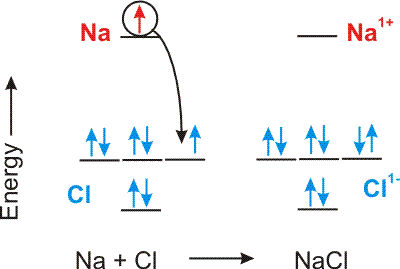
Figure 4.1: Ionic Bond Formation
4.1-3. Ionic Structure
Ionic compounds are arrays of individual ions with no identifiable molecules.
- Viewing the Video
-
•View the video in this window by selecting the play button.
-
•Use the video controls to view the video in full screen.
4.1-4. Ionic Compounds vs. Covalent Compounds
Compounds between nonmetals are covalent.
Covalent compounds are arrays of individual molecules, i.e., they are molecular.
4.1-5. Ionic or Covalent Exercise
Exercise 4.1:








Indicate whether each of the following is ionic or covalent.
-
CaCl2
-
ionic CaCl2 contains a metal and a nonmetal, so it is ionic.
-
covalent

-
SF2
-
ionic
-
covalent SF2 contains only nonmetals.

-
KCl
-
ionic KCl contains a metal and a nonmetal, so it is ionic.
-
covalent

-
CCl4
-
ionic
-
covalent CCl4 contains only nonmetals.

-
Na2O
-
ionic Na2O contains a metal and a nonmetal, so it is ionic.
-
covalent

-
F2O
-
ionic
-
covalent F2O contains only nonmetals.

-
N2O3
-
ionic
-
covalent N2O3 contains only nonmetals.

-
Fe2O3
-
ionic Fe2O3 contains a metal and a nonmetal, so it is ionic.
-
covalent

4.2 Naming Ions and Predicting their Charge
Introduction
Most ionic compounds form between a metal cation and a nonmetal anion. In this section, we examine the electrons that are lost by the metal and the orbitals that are filled by the nonmetal. Once we know which electrons are lost and which orbitals are filled, we can predict the probable charges on the ions and write their electron configurations.Prerequisites
-
•3.1 Valence Electrons (Write valence electron configurations for atoms.)
-
•3.5 Ionization Energy (Predict relative ionization energies of atoms.)
-
•3.6 Electronegativity (Predict relative electronegativities of atoms.)
Objectives
-
•Predict the cation that a metal is likely to adopt and write its electron configuration.
-
•Predict the charge on the anion that a nonmetal is likely to adopt and write its electron configuration.
-
•Determine the orbital occupancy of an ion given the charge on the ion and the occupancy of its atom.
4.2-1. Metal Cations
Cations are produced by the loss of valence electrons with those with the highest n quantum number being lost first. Consequently, first row transition metals lose their 4s electrons before they lose any 3d electrons.
-
•Monatomic cations with charges greater than +3 do not form.
-
•Electrons from the outermost shell (highest n quantum number) are lost first. This is important in determining the cations formed by transition metals.
-
•Within a shell, electrons from the subshell with the highest l quantum number are lost first. This is important for the heavier metals in Groups 3, 4, and 5.
| Group 1A & 2A Metals | Group 3A Metals |
| Lose their valence electrons to form +1 and +2 ions, respectively. | Lose all of their valence electrons to form +3 ions. Tl forms both +3 and +1 ions, but not a +2 ion. The reason is that the heavier main group elements can lose only a portion of their valence shell. Tl is 6s2 6p1. Both valence sublevels are in the same level, so the one with the highest l quantum number is emptied first. Thus, Tl can lose the 6p and not the 6s to form the +1 ion, but it cannot lose the 6s and not the 6p to form a +2 ion. |
| Group 4A Metals | Transition Metals |
| +4 monatomic ions do not exist, so the Group 4A metals cannot lose all of their valence electrons. However, the heavier metals in the group (Sn and Pb) can lose the electrons in the sublevel with the highest l quantum number, the outermost p sublevel, to form +2 ions. | Lose electrons in the level with the highest n quantum number. Thus, most transition elements lose their outermost s electrons to form +2 ions. Scandium is an exception because it loses all three valence electrons to form Sc3+ (no +2 ion). Silver forms only a +1 ion, and copper forms both +2 and +1 ions. In addition, several transition metals form a +3 ion in addition to a +2 ion. |
Table 4.1: Cations Formed by Metals
4.2-2. Nonmetal Anions
Nonmetals form anions by gaining the number of electrons required to fill their valence shell (outermost s and p sublevels).
| 7A | 6A | 5A | 4A |
| –1 ions | –2 ions | –3 ions | Monatomic anions with charges of –4 do not exist, so the Group 4A nonmetals do not form anions. |
Table 4.2: Anions Formed by Nonmetals
4.2-3. Predicting Charge and Electron Configuration Exercise
Exercise 4.2:
Each atom forms only one monatomic ion. What is the charge on that ion? (Express your answers as charge followed by magnitude. For example: +2, not 2+.)
S
-2_0__
S has six valence electrons, so it needs two more to fill its valence shell. Adding two more electrons would produce the S2– ion.

Mg
+2_0__
Mg is a 2A metal, so it loses its two valence electrons to produce the Mg2+ ion.

Sc
+3_0__
Sc is a 3A metal, so it loses its three valence electrons to produce the Sc3+ ion.

P
-3_0__
P has five valence electrons, so it needs three more to fill its valence shell. Adding three more electrons would produce the P3– ion.

Ag
+1_0__
Ag is a transition metal, but it is an important exception to the +2 ion rule that applies to most transition metal ions. Ag forms only the +1 ion.

Zn
+2_0__
The d-block of Zn is full, so it does not lose any d electrons. Thus, Zn forms only the +2 ion by losing its 4s electrons.

What are the electron configurations of the following ions? Use noble gas cores. (Separate all terms by a single space and indicate all superscripts with a carat (^). For example, [He] 2s^2 2p^2 for [He] 2s2 2p2.)
Sc3+
o_[Ar]_s
Scandium loses all of its valence electrons to become isoelectronic with argon.

N3–
o_[Ne]_s
Nitrogen gains three electrons to become isoelectronic with neon.

Ni2+
o_[Ar] 3d^8_s
Ni2+ is [Ar] 3d8. Remember that the electrons with the highest n quantum number are lost first. Thus, nickel loses its two 4s, not the 3d, electrons, to form the +2 ion.

Bi3+
o_[Xe] 6s^2 4f^14 5d^10_s
Bi3+ is [Xe] 6s2 4f14 5d10. The valence electron configuration of bismuth is 6s2 6p3. Recall that when electrons in the outermost shell are in two different sublevels, those with the highest l quantum number are lost first, so the three 6p electrons are lost before the 6s electrons.

K1+
o_[Ar]_s
K1+ is [Ar] as the 4s electron is lost to produce the cation.

Br1–
o_[Kr]_s
Br1– is [Kr]. Monatomic anions are almost always isoelectronic with a noble gas because they almost always fill their valence shell.

4.2-4. Orbital Occupancies of Ions Exercise
Exercise 4.3:




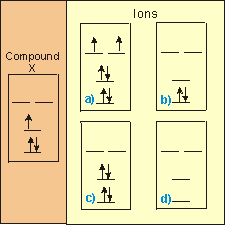
Substances can gain and/or lose electrons, and we will frequently have to consider the orbital energy diagrams of the resulting species. Consider the following example in which you must identify which ions of substance X (shown with brown background) are represented by Figures a–d.
-
Figure a
-
X4– Recount the number of additional electrons. Remember that the neutral atom has three valence electrons.
-
X3– This species has three more electrons than the compound, so it is the –3 ion. Recount the number of additional electrons. Remember that the neutral atom has three valence electrons. This species contains more electrons than the compound, so it must be an anion, i.e., it must have negative charge.
-
X1– Recount the number of additional electrons. Remember that the neutral atom has three valence electrons.
-
X1+ This species contains more electrons than the compound, so it must be an anion, i.e., it must have negative charge.
-
X2+ This species contains more electrons than the compound, so it must be an anion, i.e., it must have negative charge.
-
X3+ This species contains more electrons than the compound, so it must be an anion, i.e., it must have negative charge.

-
Figure b
-
X4– This species has fewer electrons than the compound, so it must be a cation, i.e., it must have a positive charge.
-
X3– This species has fewer electrons than the compound, so it must be a cation, i.e., it must have a positive charge.
-
X1– This species has fewer electrons than the compound, so it must be a cation, i.e., it must have a positive charge.
-
X1+ This species contains one less electron than the compound, so it is the +1 ion. This species has fewer electrons than the compound, so it must be a cation, i.e., it must have a positive charge. Recount the number of electrons lost. Recall that the neutral compound has three valence electrons.
-
X2+ Recount the number of electrons lost. Recall that the neutral compound has three valence electrons.
-
X3+ Recount the number of electrons lost. Recall that the neutral compound has three valence electrons.

-
Figure c
-
X4– Recount the number of additional electrons. Remember that the neutral atom has three valence electrons.
-
X3– Recount the number of additional electrons. Remember that the neutral atom has three valence electrons.
-
X1– This species has one more electron than the compound, so it is the –1 ion. Recount the number of additional electrons. Remember that the neutral atom has three valence electrons. This species contains more electrons than the compound, so it must be an anion, i.e., it must have negative charge.
-
X1+ This species contains more electrons than the compound, so it must be an anion, i.e., it must have negative charge.
-
X2+ This species contains more electrons than the compound, so it must be an anion, i.e., it must have negative charge.
-
X3+ This species contains more electrons than the compound, so it must be an anion, i.e., it must have negative charge.

-
Figure d
-
X4– This species has fewer electrons than the compound, so it must be a cation, i.e., it must have a positive charge.
-
X3– This species has fewer electrons than the compound, so it must be a cation, i.e., it must have a positive charge.
-
X1– This species has fewer electrons than the compound, so it must be a cation, i.e., it must have a positive charge.
-
X1+ Recount the number of electrons lost. Recall that the neutral compound has three valence electrons.
-
X2+ Recount the number of electrons lost. Recall that the neutral compound has three valence electrons.
-
X3+ This species contains three fewer electrons than the compound, so it is the +3 ion. Recount the number of electrons lost. Recall that the neutral compound has three valence electrons. This species has fewer electrons than the compound, so it must be a cation, i.e., it must have a positive charge.

4.3 Ionic vs. Atomic Size
Introduction
We saw in the previous chapter that the size of an atom depends upon the size of its outermost orbitals, and ions form when electrons leave or enter those orbitals. Consequently, the sizes of ions are different than those of the atoms. In this section, we compare the sizes of atoms, anions, and cations.Prerequisites
-
•3.3 Relative Atomic Size (Predict relative sizes of atoms from their positions in the periodic table.)
Objectives
-
•Explain why cations are smaller than their atoms, but anions are larger.
-
•Predict the relative sizes of a series of ions.
4.3-1. Relative Ion Size
Cations are smaller than their atoms, but anions are larger than their atoms.
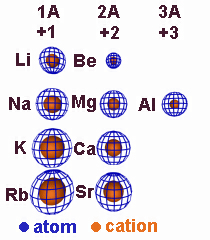
Figure 4.2
Relative Sizes of Cations and Their Atoms: A loss of electrons increases Zeff and, if the valence shell is emptied, decreases the n quantum number. Consequently, cations are smaller than their parent atoms.
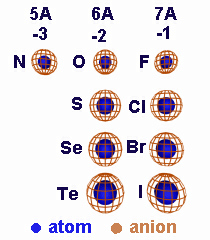
Figure 4.3
Relative Sizes of Anions and Their Atoms: A gain of electrons decreases Zeff, so anions are larger than their atoms.
4.3-2. Ionic Size Exercise
Exercise 4.4:



-
Cl1– Ionic radii of ions with the same charge increase going down a group.
-
F1– Ionic radii of ions with the same charge increase going down a group.
-
I1–

-
Na1+ Ionic radii increase in a period as the charge becomes more negative. The outermost electrons in the two metal ions are in the 2p sublevel, while those in the anion are in the 3p.
-
S2–
-
Al3+ Ionic radii increase in a period as the charge becomes more negative. The outermost electrons in the two metal ions are in the 2p sublevel, while those in the anion are in the 3p.

-
Br1–
-
K1+ K1+ is isoelectronic with Ar, and Br1– is isoelectronic with Kr. Br1– is larger than Se because the Br1– ion has more electrons than protons, so the outermost electrons are shielded very well.
-
Se K1+ is isoelectronic with Ar, and Br1– is isoelectronic with Kr. Br1– is larger than Se because the Br1– ion has more electrons than protons, so the outermost electrons are shielded very well.

4.4 Oxidation States
Introduction
Electron counting (keeping track of where the electrons are in a compound) is a valuable aid in predicting the formulas of compounds, balancing certain types of chemical equations, predicting properties, and even predicting reactive centers. In this lesson, we introduce the oxidation state method for counting electrons. We then show how to determine the oxidation states of an atom in a molecule or ion and how to use oxidation states to predict formulas.Prerequisites
-
•3.4 Relative Orbital Energies (Predict relative valence orbital energies of atoms.)
-
•3.5 Ionization Energy (Predict relative valence orbital energies of atoms based on their ionization energies.)
-
•3.6 Electronegativity (Predict relative valence orbital energies of atoms based on their electronegativities.)
-
•2.3 Bohr Model
Objectives
-
•Define the term "oxidation state."
-
•Explain how the charge on a species (compound or ion) is related to the oxidation states of its atoms.
-
•Determine the oxidation states of the atoms in an ion or molecule.
-
•Predict chemical formulas for binary compounds.
4.4-1. Oxidation States from Chemical Formulas Video
- Viewing the Video
-
•View the video in this window by selecting the play button.
-
•Use the video controls to view the video in full screen.
4.4-2. Oxidation State Definition
The oxidation state of an atom in a compound is the charge it would have if its bonds were ionic.
-
•negative if it is the more electronegative atom, and
-
•positive if it is the less electronegative atom.
4.4-3. Oxidation States of Hydrogen and Chlorine
The most common oxidation state of Cl is –1 because most of its bonds are to less electronegative elements.
Cl has a zero oxidation state when bound to itself, and it can have positive oxidation states when bound to O or F.
| Ox State of Cl | Examples |
| –1 | CCl4O, NCl3, KCl |
| 0 | Cl2 |
| +1 | ClF, Cl2O, ClO1– |
| +3 | ClF3, ClO21– |
| +5 | ClO31– |
| +7 | ClO41– |
Table 4.3
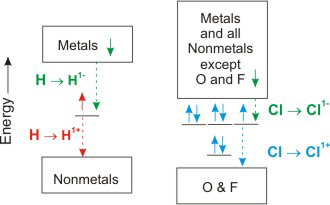
Figure 4.4
Oxidation States of Hydrogen and Chlorine
4.4-4. Oxidation State Sum
The oxidation states of all of the atoms in a chemical species must sum to the charge on the species.
-
•the oxidation states of all the atoms in an ion must sum to the charge of the ion, and
-
•the oxidation states of all the atoms in a molecule must sum to zero.
Example:
For example, let us determine the charge on the carbonate ion (CO3x) given that the oxidation states of C and O are +4 and –2, respectively. The charge on the ion equals the sum of the oxidation states, so we write
The carbonate ion is the CO32– ion.
For example, let us determine the charge on the carbonate ion (CO3x) given that the oxidation states of C and O are +4 and –2, respectively. The charge on the ion equals the sum of the oxidation states, so we write
charge = (1C)(+4/C) + (3O)(−2/O) = 4 − 6 = −2
4.4-5. Oxidation State Guidelines
The oxidation state of an atom lies between its group number and its group number minus eight.
-
•The Group 1A, 2A, and 3A metals are usually found in their highest oxidation states (+1, +2, and +3) when bound to nonmetals.
-
•Transition elements tend to adopt oxidation states of +2 and +3, but Ag is +1 and Cu is +1 or +2 in most of their compounds. Some transition elements can achieve oxidation states as high as their group number when surrounded by highly electronegative atoms (usually oxygen). For example, vanadium is +5 in VO43–, chromium is +6 in CrO42–, and Mn is +7 in MnO41–.
4.4-6. Oxidation State Rules
The oxidation state guidelines give us ranges for the oxidation states of the elements, but many atoms have the same oxidation state in almost all of their compounds. To determine the oxidation state of an atom in a molecule or ion, use the oxidation state rules given in Table 4.4. The oxidation state rules are listed in order of priority, so they should be used in the order given; that is, a rule takes precedence over any rule below it, or any rule can be violated only to satisfy a rule above it. Note that the oxidation state rules merely reflect what we already know about atomic properties: atoms with low ionization energies (Groups 1A and 2A) are generally found in the highest oxidation states, while highly electronegative atoms (F and O) are generally in their lowest oxidation states.| Rule | Reason |
| The oxidation states of the atoms in an element are all zero. | When the valence orbitals of the two atoms are the same, the bonding electrons are assumed to be shared, not transferred. For example, the oxidation state of Cu in metallic Cu is zero, both F atoms in F2 are zero (the only time F is not –1), and all eight sulfur atoms in S8 are zero. |
| F is –1. | The valence orbitals of F are lower than the valence orbitals on any other element, i.e., F is the most electronegative element. Consequently, it is always assigned the bonding electrons. There is only one compound in which an F atom is not –1. What is it? Hint: See the only rule that takes priority over this rule. |
| 1A metals are +1, 2A metals are +2, and Al is +3. | The valence orbitals in these metals are very high in energy, i.e., these metals have low ionization energies. They also become isoelectronic with a noble gas when they form the ions. | H is +1. | The 1s orbital on H is lower in energy than the valence orbitals of most metals and higher in energy than those of most nonmetals. Therefore, H is +1 except when Rule 1 forces it to be 0 or Rule 3 (metals with higher energy valence orbitals) forces it to be –1. |
| O is –2. | Oxygen is the second most electronegative atom, so it is usually assigned the bonding electrons. However, it is not –2 when it is elemental O2 (Rule 1) or bonds to F (Rule 2). In addition, it can be –1 if Rules 3 and 4 force it. Compounds in which the oxidation state of oxygen is –1 are called peroxides. Peroxides contain O22–, which has an O–O bond. Hydrogen peroxide (H2O2) is a common peroxide. |
| 7A elements are –1. | Halogens are electronegative, so they tend to fill their valence shell to attain –1 oxidation states. However, they can attain positive oxidation states when bound to more electronegative atoms, such as oxygen, or more electronegative halogens, e.g., Cl is +7 in ClO41–, Br is +5 in BrO31–, and I is +3 in IF3. |
Table 4.4
4.4-7. Oxidation State Exercise
Exercise 4.5:
Oxidation State Rules
-
1Atoms in elements are zero.
-
2F is –1.
-
3Group 1A metals are +1, 2A metals are +2, and Al is +3.
-
4H is +1.
-
5O is –2.
-
6Group 7A elements are –1.
FeCl3
+3_0__
Cl is –1 by Rule 6, and FeCl3 has no charge, so x + 3(–1) = 0, or x = +3. The oxidation state of Fe is +3 in FeCl3. Many transition metals are found in the +3 oxidation state.

MnO41–
+7_0__
O is –2 by Rule 5, and the charge on the MnO4 1– ion is –1, so x + 4(–2) = –1, or x = +7. The 
oxidation state of Mn is +7 in MnO41–. This very high oxidation state is stabilized by the highly electronegative oxygen atoms.
Cr2O72–
+6_0__
O is –2 by Rule 5, and the charge on the Cr2O72– ion is –2, so
2x + 7(–2) = –2, or 2x = +12, or
x = +6. The oxidation state of Cr is +6 in Cr2O72–. Cr attains its highest oxidation state when surrounded by the highly electronegative oxygen atoms.
x = +6. The oxidation state of Cr is +6 in Cr2O72–. Cr attains its highest oxidation state when surrounded by the highly electronegative oxygen atoms.
CaO2
+2_0__
Ca is a Group 2A metal, so Ca has priority over oxygen and is assigned a +2 oxidation state by Rule 2. The oxidation state of O is determined as +2 + 2x = 0, or 2x = –2, or x = –1. This compound contains the O22– ion, which is the peroxide ion. Thus, CaO2 is called calcium peroxide. Calcium oxide is CaO.

NH3
-3_0__
H is +1 by Rule 4, and there is no charge on NH3, so x + 3(+1) = 0, or x = –3. The oxidation state of N is –3 in NH3. This is one of the few cases where the element with the negative oxidation state is written first. The H is not written first because hydrogen atoms written at the beginning of a formula are assumed to be acidic (Chapter 12).

4.4-8. Using Oxidation States to Determine Charge Exercise
Exercise 4.6:
Determine the charge on each ion. Use the following oxidation states. (Express your answers as charge followed by magnitude. For example: +2, not 2+.)
- O = –2 Cr = +6 P = +5 Mn = +7 N = +5
CrO4x
-2_0__
The charge must equal the sum of the oxidation states: (1 Cr)(+6/Cr) + (4 O)(–2/O) = 6 – 8 = –2. The ion is the chromate ion: CrO42–.

PO4x
-3_0__
The charge must equal the sum of the oxidation states: (1 P)(+5/Cr) + (4 O)(–2/O) = 5 – 8 = –3. The ion is the phosphate ion: PO43–.

MnO4x
-1_0__
The charge must equal the sum of the oxidation states:
(1 Mn)(+7/Mn) + (4 O)(–2/O) = 7 – 8 = –1. The ion is the permanganate ion: MnO41–.
(1 Mn)(+7/Mn) + (4 O)(–2/O) = 7 – 8 = –1. The ion is the permanganate ion: MnO41–.
NO3x
-1_0__
The charge must equal the sum of the oxidation states: (1 N)(+5/Mn) + (3 O)(–2/O) = 5 – 6 = –1. The ion is the nitrate ion: NO31–.

4.4-9. Chemical Formulas from Oxidation States Video
- Viewing the Video
-
•View the video in this window by selecting the play button.
-
•Use the video controls to view the video in full screen.
-
•View the video in text format by scrolling down.
-
•Jump to the exercises for this topic.
4.4-10. Predicting Formulas
The likely formula of a compound can be predicted by assigning likely oxidation states to each of the atoms.
-
1Assign oxidation states to the two elements. If the elements are not listed in the oxidation state rules, assign +Group number to the less electronegative element and –(8 – Group number) to the more electronegative element. However, recall that nonmetals can empty only their p sublevel, leaving two s electrons. In this case, OXpos = Group number – 2.
-
2Determine the ratio of oxidation states as a ratio of smallest whole numbers.
-
3The ratio of the subscripts in the formula varies inversely with the ratio of oxidation states, so the whole numbers in step 1 are the subscripts.
-
4Write the formula, but be sure to always write the less electronegative element first.
4.4-11. Predicting Formulas Exercise
Exercise 4.7:
Predict the formula of the compound formed between each pair of elements below. (Indicate any subscripted characters with an underscore (_) and any superscripted characters with a carat (^). For example, NH_4^1+ for NH41+.)
oxidation state of 1st element
Na and O
+1_0__
Na is a Group 1A metal and is always found in the +1 oxidation state in its compounds.

Zn and Cl
+2_0__
Zn is a Group 2B metal and is always found in the +2 oxidation state in its compounds.

Al and S
+3_0__
Al is a Group 3A metal and is always found in the +3 oxidation state in its compounds.

C and O
+4_0__
C is the less electronegative element, so we assume that it is in its highest oxidation state. It is a Group 4A nonmetal, so its highest oxidation state is +4.

oxidation state of 2nd element
Na and O
-2_0__
Oxygen is usually found in the –2 oxidation state in its compounds.

Zn and Cl
-1_0__
Chlorine is usually found in the –1 oxidation state in its compounds.

Al and S
-2_0__
Sulfur is usually found in the –2 oxidation state in its compounds.

C and O
-2_0__
Oxygen is usually found in the –2 oxidation state in its compounds.

LCM of the two oxidation states
Na and O
2_0__
The lowest common multiple of 2 and 1 is 2.

Zn and Cl
2_0__
The lowest common multiple of 2 and 1 is 2.

Al and S
6_0__
The lowest common multiple of 3 and 2 is 6.

C and O
4_0__
The lowest common multiple of 4 and 2 is 4.

formula of compound
Na and O
o_Na_2O_s
The LCM = 2, so two +1 atoms and one –2 atom are required. Sodium oxide is Na2O.

Zn and Cl
o_ZnCl_2_s
The LCM = 2, so one +2 atom and two –1 atoms are required. Zinc chloride is ZnCl2.

Al and S
o_Al_2S_3_s
The LCM = 6, so two +3 atoms and three –2 atoms are required. Aluminum sulfide is Al2S3.

C and O
o_CO_2_s
The LCM = 4, so one +4 atom and two –2 atoms are required. The compound formed between carbon and oxygen is CO2.

4.4-12. Writing Formulas of Binary Compounds Exercise
Exercise 4.8:
Use the highest and lowest oxidation states of nonmetals and common oxidation states of metals to predict the formulas of the compounds that would form between each pair of elements. (Indicate any subscripted characters with an underscore (_) and any superscripted characters with a carat (^). For example, NH_4^1+ for NH41+.)
phosphorus and chlorine
o_PCl_5_s
Phosphorus is a 5A element and chlorine is a 7A. Chlorine is more electronegative, so it is assigned the lowest oxidation state (7 – 8 = –1), while phosphorus is assigned its highest oxidation state (+5). The lowest common multiple is 5, so the compound is PCl5. Phosphorus also forms a +3 oxidation state, in which case, the compound would be PCl3

calcium and oxygen
o_CaO_s
Calcium is a 2A metal, so it is +2. Oxygen is a 6A nonmetal, so it is assigned an oxidation state of –2. The compound is CaO.

carbon and fluorine
o_CF_4_s
Fluorine is the most electronegative element, so it is always assigned the negative oxidation state. Thus, F is –1. Carbon is a 4A element, so it is assigned a +4 oxidation state when combined with fluorine. Four fluorine atoms at –1 each are required to balance the +4 carbon atom, so the formula is CF4.

copper and sulfur
o_CuS_s
Copper is a transition element, and it commonly adopts the +2 oxidation state. Sulfur is a 6A nonmetal, so its oxidation state is –2. The formula is CuS. However, copper also adopts the +1 oxidation state, in which case, the formula would be Cu2S.

4.4-13. Orbital Diagrams and Oxidatation State Exercise
Exercise 4.9:
Use the energy level diagram for the valence electrons of X, Y, and Z to determine each answer. Always write the more electronegative element last. (Indicate any subscripted characters with an underscore (_) and any superscripted characters with a carat (^). For example, NH_4^1+ for NH41+. When more than one oxidation state is possible, choose the highest oxidation state.)
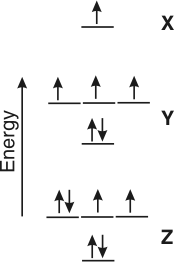

oxidation state of X when it bonds to Y or Z
1_0__
The electron on X is at higher energy than the unfilled orbitals on Y and Z, so the electron would be lost to form a +1 ion.

oxidation state of Y when it bonds to X
-3_0__
The unfilled orbitals on Y are lower in energy than the electrons on X, so it would gain three electrons from X to fill its valence shell.

oxidation state of Y when it bonds to Z
5_0__
The electrons on Y are at higher energy than the unfilled orbitals on Z, so it would either lose the three p electrons or all five electrons.

oxidation state of Z when it bonds to Y
-2_0__
The unfilled orbitals on Z are at the lowest energy, so three electrons would be donated to Z from either X or Y to fill its valence shell.

formula of the compound formed between X and Y
o_X_3Y_s
X is +1 and Y is –3, so the compound is X3Y.

formula of the compound formed between X and Z
o_X_2Z_s
X is +1 and Z is –2, so the compound is X2Z.

formula of the compound formed between Y and Z
o_Y_2Z_5_s
Z is –2 and Y is either +3 or +5. Y is less electronegative, so it is written first. If Y is +3, the compound is Y2Z3, and if it is +5, the compound is Y2Z5.

What are the identities (symbols) of X, Y, and Z if they are all in the second period?
X =
o_Li_s
X has only one valence electron, so it is in Group 1A. X is Li.

Y =
o_N_s
Y has five valence electrons, so it is in Group 5A and the second period. It is N.

Z =
o_O_s
Z has six valence electrons, so it is in Group 6A and the second period. It is O.

4.5 Polyatomic Ions
Introduction
So far, we have considered only monatomic ions (ions composed of a single atom), but many ions consist of several atoms and are called polyatomic ions. The bonds between the atoms in a polyatomic ion are not ionic, but a polyatomic ion does have a net charge and bonds to other ions via an ionic bond.Objectives
-
•Identify the common polyatomic ions.
4.5-1. Polyatomic Ions
A number of ionic compounds are composed of polyatomic ions, which are charged groups of covalently bound atoms. While the polyatomic ion forms ionic bonds with oppositely charged ions, the atoms within a polyatomic ion are nonmetals held together by covalent bonds. Many of the polyatomic ions are oxoanions, i.e., they are negative ions that contain oxygen atoms covalently bound to another element. In the common polyatomic ions listed in Table 4.5, the only cations are ammonium and hydronium, and the only anions that are not oxoanions are hydroxide and cyanide. Recall that ionic compounds can be identified as those that contain metals because metals represent almost all of the common monatomic cations. However, ions can also be polyatomic. Ammonium is by far the most common polyatomic cation in compounds. Thus, NH4Cl, NH4NO3, and (NH4)2SO4 are also ionic compounds. We conclude that ionic compounds are those that contain either a metal or a polyatomic cation such as ammonium.| Cations | ||||
| NH41+ | ammonium ion | H3O1+ | hydronium ion | |
| Anions | ||||
| C2H3O21– | acetate ion | OH1– | hydroxide ion | |
| CO32– | carbonate ion | NO31– | nitrate ion | |
| ClO41– | perchlorate ion | NO21– | nitrite ion | |
| ClO31– | chlorate ion | MnO41– | permanganate ion | |
| ClO21– | chlorite ion | O22– | peroxide ion | |
| ClO1– | hypochlorite ion | PO43– | phosphate ion | |
| CrO42– | chromate ion | SO42– | sulfate ion | |
| Cr2O72– | dichromate ion | SO32– | sulfite ion | |
| CN1– | cyanide ion | |||
Table 4.5: Some Common Polyatomic Ions
4.6 Naming Ionic Compounds
Introduction
Understanding how ionic compounds are named helps you better communicate about chemistry. In this section, we explain how binary ionic compounds and ionic compounds containing polyatomic ions are named.Prerequisites
Objectives
-
•Name ions that contain one or more protons.
-
•Name ionic compounds containing polyatomic ions.
4.6-1. Binary Compounds
The oxidation state of the metal is given as a Roman numeral in parentheses when naming inorganic compounds that contain a metal that can attain more than one oxidation state.
-
•NaCl = sodium chloride (Group 1A are always +1.)
-
•Mg3N2 = magnesium nitride (Group 2A are always +2.)
-
•ZnBr2 = zinc bromide (Zn is always +2.)
-
•AgF = silver fluoride (Ag is always +1.)
-
•Sc2O3 = scandium oxide (Sc is always +3.)
-
•CoO is cobalt(II) oxide to distinguish it from Co2O3, which is cobalt(III) oxide.
-
•TlCl is thallium(I) chloride to distinguish it from TlCl3, which is thallium(III) chloride.
4.6-2. Naming Exercise
Exercise 4.10:
Name the following compounds.
AlBr3
o_aluminum bromide_s
Al is always +3.

ZnCl2
o_zinc chloride_s
Zn is always +2.

Ag2O
o_silver oxide_s
Ag is always +1.

FeCl3
o_iron(III) chloride_s
Fe can be +2 or +3.

CuCl
o_copper(I) chloride_s
Cu can be +1 or +2.

PbO2
o_lead(IV) oxide_s
Pb can be +2 or +4.

Hg2Cl2
o_mercury(I) chloride_s
Hg can be +1 or +2.

MnO2
o_manganese(IV) oxide_s
Manganese can adopt many oxidation states.

ZnBr2
o_zinc bromide_s
Zinc is always in the +2.

SnO2
o_tin(IV) oxide_s
Tin can be +2 and +4.

CoCl3
o_cobalt(III) chloride_s
Cobalt is a transition metal that can adopt several oxidation states.

K2O
o_potassium oxide_s
Potassium is a 1A metal, so it can attain only the +1 oxidation state.

4.6-3. Naming Oxoanions of the Elements of Groups 4, 5, and 6
Suffixes are used to indicate the oxidation state of the central atom in most oxoanions:-
•-ate implies that the central atom is in its highest oxidation state (group number).
-
•-ite tells us that the oxidation state of the central atom is lower than the highest oxidation state by two because the ion has one less oxygen atom than the corresponding ion that ends in -ate.
| Group 4 | CO32– is the carbonate ion because Group 4A carbon is in the +4 oxidation state. |
| Group 5 | PO43– is the phosphate ion because Group 5A phosphorus is in the +5 oxidation state. However, NO31– is the nitrate ion. Removing a single oxygen atom produces NO21–, the nitrite ion. |
| Group 6 | SO42– is the sulfate ion because Group 6A sulfur is in the +6 oxidation state. Removal of one oxygen atom produces the sulfite ion, SO32–. |
Table 4.6
4.6-4. Naming Oxoanions of Group 7
The oxoanions of the Group 7A elements are an exception because, unlike the others, they each form four oxoanions. Consequently, both prefixes and suffixes must be used (see Table 4.7). In the perchlorate ion (ClO41–), the chlorine is in its highest oxidation state (+7). The chlorate ion (ClO31–) has one less oxygen, so the oxidation state of Cl is two less, or +5. The chlorite ion (ClO21–) has one less oxygen than chlorate, which lowers the oxidation state of Cl to +3. Finally, the hypochlorite ion (ClO1–) has one less oxygen than chlorite, and the oxidation state of the Cl is reduced to +1. Similarly, perbromate is BrO41–, bromate is BrO31–, etc. Note, however, that fluorine is the most electronegative element, so it never has a positive oxidation state. Consequently, fluorine forms no oxoanions.| Prefix | Suffix | Oxidation State of Halogen |
Formula |
| Per- | -ate | +7 | XO41– |
| -ate | +5 | XO31– | |
| -ite | +3 | XO21– | |
| Hypo- | -ite | +1 | XO1– |
Table 4.7: Prefixes and Suffixes of the Oxoanions Formed by the Halogens
4.6-5. Protonated Ions
Ions that have charges of –2 and –3 pick up protons to produce protonated anions, which are named by placing hydrogen (or dihydrogen) and a space in front of the name of the ion. An older, but still common, method for naming some of these ions is to replace the "hydrogen and a space" with simply "bi" with no space. Thus, HS1– is either the hydrogen sulfide ion or the bisulfide ion.| HCO31– | hydrogen carbonate or bicarbonate ion |
| HPO42– | hydrogen phosphate ion |
| H2PO41– | dihydrogen phosphate ion |
| HSO41– | hydrogen sulfate or bisulfate ion |
| HSO31– | hydrogen sulfite or bisulfite ion |
Table 4.8: Common Protonated Oxoanions
4.6-6. Writing Formulas for Compounds with Polyatomic Ions Exercise
Exercise 4.11:
Use the fact that the names of the polyatomic ions are used without change when naming compounds that contain one or more polyatomic ions to write formulas for the following compounds. (Indicate any subscripted characters with an underscore (_) and any superscripted characters with a carat (^). For example, NH_4^1+ for NH41+.)
ammonium bromide
o_NH_4Br_s
The ammonium ion is NH41+. The bromide ion is Br1–. Remember that the ending is -ate or -ite for oxoanions. The charges cancel in the 1:1 compound, so ammonium bromide is NH4Br.

potassium chlorate
o_KClO_3_s
The potassium (Group 1A) ion is K1+. The chlorate ion is ClO31–. The charges cancel in the 1:1 compound, so potassium chlorate is KClO3.

cobalt(III) nitrate
o_Co(NO_3)_3_s
The cobalt(III) ion is Co3+. The nitrate ion is NO31–. Three nitrate ions are required for each cobalt(III) ion, so cobalt(III) nitrate is Co(NO3)3.

scandium phosphate
o_ScPO_4_s
The scandium ion is Sc3+. The phosphate ion is PO43–. The +3 and –3 charges cancel in a 1:1 ratio, so scandium phosphate is ScPO4.

ammonium sulfate
o_(NH_4)_2SO_4_s
The ammonium ion is NH41+. The sulfate ion is SO42–. Two ammonium ions are required to balance the charge of one sulfate ion, so the formula of ammonium sulfate is (NH4)2SO4.

calcium cyanide
o_Ca(CN)_2_s
The calcium ion is Ca2+. The cyanide ion is CN1–. Note that this is one of only two anions that end in -ide but are not monatomic ions. The other is the hydroxide ion, OH1–. The formula of calcium cyanide is Ca(CN)2.